Combined effect of propranolol, vincristine and bevacizumab on HUVECs and BJ cells
- PMID: 30651796
- PMCID: PMC6307438
- DOI: 10.3892/etm.2018.6925
Combined effect of propranolol, vincristine and bevacizumab on HUVECs and BJ cells
Abstract
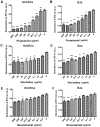
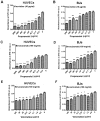
Infantile hemangioma is one of the most common benign tumors affecting children, with ~10-15% requiring medical treatment. These tumors consist of endothelial cells and stromal components, including fibroblasts, pericytes and mast cells. Effects of propranolol treatment in combination with bevacizumab or vincristine on cell growth were compared in the current study using human umbilical vein endothelial cells (HUVECs) and BJ human normal fibroblasts (BJs) to determine potential synergic effects in vitro. Inhibition of cell growth was investigated using MTT assays and cytotoxicity of the drugs in various combinations was expressed as half inhibitory concentration (IC50). Apoptosis was investigated using flow cytometry, with Alexa Fluor 488 and propidium iodide. Propranolol inhibited BJ and HUVEC growth in a dose-dependent manner, with increased response observed in BJs (IC50, 148,32 µg/ml; standard error logIC50, 0.07). Treatment with vincristine induced the strongest growth inhibition in HUVECs (IC50, 17,89 µg/ml; standard error log IC50, 0.07) and BJs (IC50, 24,81 µg/ml; standard error log IC50, 0.08) compared with propranolol (HUVEC IC50, 81,94 µg/ml; standard error log IC50, 0.06; BJ-IC50, 148,32 µg/ml; standard error logIC50, 0.07) or bevacizumab (HUVEC IC50 96,91 µg/ml; standard error log IC50, 0.06; BJ IC50, 182,70 µg/ml; standard error log IC50, 0.09) alone. Bevacizumab was the weakest cytotoxic agent. Combination treatment of vincristine with bevacizumab induced the highest levels of apoptosis in HUVECs compared with all other treatments and triple-drug therapy induced the levels of apoptosis in BJs. Single treatment with vincristine, propranolol or bevacizumab induced apoptosis in BJs and HUVECs. In BJs, triple treatment exhibited the greatest influence on apoptosis, compared with single and dual treatments and in HUVECs, vincristine and bevacizumab combination treatment induced apoptosis to the highest level. The present study offers novel perspectives in drug repurposing studies for the three drugs, particularly in diseases where the pathogenesis is based on healthy endothelial cell proliferation, including hemangiomas.
Keywords: bevacizumab; fibroblasts; human umbilical vein endothelial cells; infantile hemangioma; propranolol; vincristine.
Figures



References
LinkOut - more resources
Full Text Sources
