Bifidobacterium lactis Ameliorates the Risk of Food Allergy in Chinese Children by Affecting Relative Percentage of Treg and Th17 Cells
- PMID: 30651897
- PMCID: PMC6311867
- DOI: 10.1155/2018/4561038
Bifidobacterium lactis Ameliorates the Risk of Food Allergy in Chinese Children by Affecting Relative Percentage of Treg and Th17 Cells
Abstract
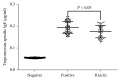
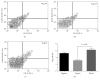
We aimed to explore the therapeutic effect of Bifidobacterium lactis on food allergy by investigating the percentage of Treg and Th17 cells in Chinese children and related molecular mechanisms. A total of 256 children with food allergy were evenly assigned into two groups: BG, the children received 10 ml B. lactis (1 × 106/ml) daily, and CG, the children received the solution without B. lactis daily for three months. Allergic symptoms, serum IgE, and food antigen-specific IgE were measured. A mouse allergy model was established by using shrimp tropomyosin and treated with B. lactis. Relative mRNA levels of Treg- and Th17-associated cytokines were measured by using quantitative PCR. The percentage of Treg and Th17 cells in spleen were measured by using flow cytometry. After 3-month therapy, the allergic symptoms of the BG were remarkably reduced when compared with the CG (P < 0.05). Serum levels of IgE and food antigen-specific IgE were decreased too (P < 0.05). Similar results were also found in a mouse allergy model. After B. lactis treatment, the relative mRNA level of FoxP3 was significantly enhanced in the B. lactis therapy group when compared to positive controls. In addition, relative mRNA levels of FoxP3 and TGF-β associated with Treg cells were increased, whereas relative mRNA levels of IL-17A and IL-23 associated with Th17 were reduced. B. lactis treatment significantly increased the ratio of Treg and Th17 cells in a mouse allergy model (P < 0.05). B. lactis effectively alleviates allergic symptoms by increasing the ratio of Treg and Th17 cells.
Figures





References
-
- Lomidze N., Gotua M. Prevalence of self-reported food allergy in different age groups of Georgian population. Georgian Medical News. 2015;241:40–44. - PubMed
LinkOut - more resources
Full Text Sources

