Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential
- PMID: 30651940
- PMCID: PMC6327926
- DOI: 10.1080/20013078.2018.1560809
Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential
Abstract
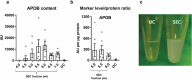
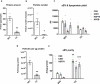
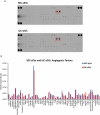
Interest in small extracellular vesicles (sEVs) as functional carriers of proteins and nucleic acids is growing continuously. There are large numbers of sEVs in the blood, but lack of standardised methods for sEV isolation greatly limits our ability to study them. In this report, we use rat plasma to systematically compare two commonly used techniques for isolation of sEVs: ultracentrifugation (UC-sEVs) and size-exclusion chromatography (SEC-sEVs). SEC-sEVs had higher particle number, protein content, particle/protein ratios and sEV marker signal than UC-sEVs. However, SEC-sEVs also contained greater amounts of APOB+ lipoproteins and large quantities of non-sEV protein. sEV marker signal correlated very well with both particle number and protein content in UC-sEVs but not in all of the SEC-sEV fractions. Functionally, both UC-sEVs and SEC-sEVs isolates contained a variety of proangiogenic factors (with endothelin-1 being the most abundant) and stimulated migration of endothelial cells. However, there was no evident correlation between the promigratory potential and the quantity of sEVs added, indicating that non-vesicular co-isolates may contribute to the promigratory effects. Overall, our findings suggest that UC provides plasma sEVs of lower yields, but markedly higher purity compared to SEC. Furthermore, we show that the functional activity of sEVs can depend on the isolation method used and does not solely reflect the sEV quantity. These findings are of importance when working with sEVs isolated from plasma- or serum-containing conditioned medium.
Keywords: Exosomes; angiogenesis; blood; endothelial cells; endothelin-1; lipoproteins; plasma; vesicle purification.
Figures







References
-
- Lawson C, Vicencio JM, Yellon DM, et al. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–R71. - PubMed
-
- van Niel G, D’Angelo G, Raposo G.. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–18. - PubMed
-
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

