A specific synbiotic-containing amino acid-based formula in dietary management of cow's milk allergy: a randomized controlled trial
- PMID: 30651972
- PMCID: PMC6332540
- DOI: 10.1186/s13601-019-0241-3
A specific synbiotic-containing amino acid-based formula in dietary management of cow's milk allergy: a randomized controlled trial
Abstract
Background: Here we report follow-up data from a double-blind, randomized, controlled multicenter trial, which investigated fecal microbiota changes with a new amino acid-based formula (AAF) including synbiotics in infants with non-immunoglobulin E (IgE)-mediated cow's milk allergy (CMA).
Methods: Subjects were randomized to receive test product (AAF including fructo-oligosaccharides and Bifidobacterium breve M-16V) or control product (AAF) for 8 weeks, after which infants could continue study product until 26 weeks. Fecal percentages of bifidobacteria and Eubacterium rectale/Clostridium coccoides group (ER/CC) were assessed at 0, 8, 12, and 26 weeks. Additional endpoints included stool markers of gut immune status, clinical symptoms, and safety assessments including adverse events and medication use.
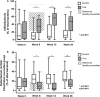
Results: The trial included 35 test subjects, 36 controls, and 51 in the healthy reference group. Study product was continued by 86% and 92% of test and control subjects between week 8-12, and by 71% and 80%, respectively until week 26. At week 26 median percentages of bifidobacteria were significantly higher in test than control [47.0% vs. 11.8% (p < 0.001)], whereas percentages of ER/CC were significantly lower [(13.7% vs. 23.6% (p = 0.003)]. Safety parameters were similar between groups. Interestingly use of dermatological medication and reported ear infections were lower in test versus control, p = 0.019 and 0.011, respectively. Baseline clinical symptoms and stool markers were mild (but persistent) and low, respectively. Symptoms reduced towards lowest score in both groups.
Conclusion: Beneficial effects of this AAF including specific synbiotics on microbiota composition were observed over 26 weeks, and shown suitable for dietary management of infants with non-IgE-mediated CMA.Trial Registration NTR3979.
Keywords: Bifidobacterium breve M-16V; Cow’s milk allergy; Gut microbiota; Prebiotic; Probiotic; Symptoms.
Figures
Similar articles
-
A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow's milk allergic infants: a randomized controlled trial.Clin Transl Allergy. 2019 May 31;9:27. doi: 10.1186/s13601-019-0267-6. eCollection 2019. Clin Transl Allergy. 2019. PMID: 31164972 Free PMC article.
-
A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants.Pediatr Res. 2018 Mar;83(3):677-686. doi: 10.1038/pr.2017.270. Epub 2017 Dec 6. Pediatr Res. 2018. PMID: 29155807 Free PMC article. Clinical Trial.
-
Tolerance development in cow's milk-allergic infants receiving amino acid-based formula: A randomized controlled trial.J Allergy Clin Immunol. 2022 Feb;149(2):650-658.e5. doi: 10.1016/j.jaci.2021.06.025. Epub 2021 Jul 2. J Allergy Clin Immunol. 2022. PMID: 34224785 Clinical Trial.
-
Amino Acid Formula Containing Synbiotics in Infants with Cow's Milk Protein Allergy: A Systematic Review and Meta-Analysis.Nutrients. 2021 Mar 14;13(3):935. doi: 10.3390/nu13030935. Nutrients. 2021. PMID: 33799379 Free PMC article.
-
The potential for pre-, pro- and synbiotics in the management of infants at risk of cow's milk allergy or with cow's milk allergy: An exploration of the rationale, available evidence and remaining questions.World Allergy Organ J. 2019 Jun 4;12(5):100034. doi: 10.1016/j.waojou.2019.100034. eCollection 2019. World Allergy Organ J. 2019. PMID: 31194186 Free PMC article. Review.
Cited by
-
Highlights and recent developments in allergic diseases in EAACI journals (2019).Clin Transl Allergy. 2020 Dec 3;10(1):56. doi: 10.1186/s13601-020-00366-3. Clin Transl Allergy. 2020. PMID: 33292572 Free PMC article. Review.
-
The Remaining Challenge to Diagnose and Manage Cow's Milk Allergy: An Opinion Paper to Daily Clinical Practice.Nutrients. 2023 Nov 13;15(22):4762. doi: 10.3390/nu15224762. Nutrients. 2023. PMID: 38004156 Free PMC article. Review.
-
The Role of the Gut Microbiome in Cow's Milk Allergy: A Clinical Approach.Nutrients. 2022 Oct 28;14(21):4537. doi: 10.3390/nu14214537. Nutrients. 2022. PMID: 36364799 Free PMC article. Review.
-
A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow's milk allergic infants: a randomized controlled trial.Clin Transl Allergy. 2019 May 31;9:27. doi: 10.1186/s13601-019-0267-6. eCollection 2019. Clin Transl Allergy. 2019. PMID: 31164972 Free PMC article.
-
Synbiotic containing extensively hydrolyzed formula improves gastrointestinal and atopic symptom severity, growth, caregiver quality of life, and hospital-related healthcare use in infants with cow's milk allergy.Immun Inflamm Dis. 2022 Jun;10(6):e636. doi: 10.1002/iid3.636. Immun Inflamm Dis. 2022. PMID: 35634950 Free PMC article.
References
-
- Bellini F, Ricci G, Remondini D, Pession A. Cow’s milk allergy (CMA) in children: identification of allergologic tests predictive of food allergy. Eur Ann Allergy Clin Immunol. 2014;46:100–105. - PubMed
-
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55:221–229. doi: 10.1097/MPG.0b013e31825c9482. - DOI - PubMed
-
- Dambacher WM, de Kort EH, Blom WM, Houben GF, de Vries E. Double-blind placebo-controlled food challenges in children with alleged cow’s milk allergy: prevention of unnecessary elimination diets and determination of eliciting doses. Nutr J. 2013;12:22. doi: 10.1186/1475-2891-12-22. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

