Coxsackievirus A10 atomic structure facilitating the discovery of a broad-spectrum inhibitor against human enteroviruses
- PMID: 30652025
- PMCID: PMC6331555
- DOI: 10.1038/s41421-018-0073-7
Coxsackievirus A10 atomic structure facilitating the discovery of a broad-spectrum inhibitor against human enteroviruses
Abstract
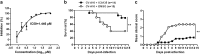
Coxsackievirus A10 (CV-A10) belongs to the Enterovirus species A and is a causative agent of hand, foot, and mouth disease. Here we present cryo-EM structures of CV-A10 mature virion and native empty particle (NEP) at 2.84 and 3.12 Å, respectively. Our CV-A10 mature virion structure reveals a density corresponding to a lipidic pocket factor of 18 carbon atoms in the hydrophobic pocket formed within viral protein 1. By structure-guided high-throughput drug screening and subsequent verification in cell-based infection-inhibition assays, we identified four compounds that inhibited CV-A10 infection in vitro. These compounds represent a new class of anti-enteroviral drug leads. Notably, one of the compounds, ICA135, also exerted broad-spectrum inhibitory effects on a number of representative viruses from all four species (A-D) of human enteroviruses. Our findings should facilitate the development of broadly effective drugs and vaccines for enterovirus infections.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Molecular basis of Coxsackievirus A10 entry using the two-in-one attachment and uncoating receptor KRM1.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18711-18718. doi: 10.1073/pnas.2005341117. Epub 2020 Jul 20. Proc Natl Acad Sci U S A. 2020. PMID: 32690697 Free PMC article.
-
A 3.0-Angstrom Resolution Cryo-Electron Microscopy Structure and Antigenic Sites of Coxsackievirus A6-Like Particles.J Virol. 2018 Jan 2;92(2):e01257-17. doi: 10.1128/JVI.01257-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093091 Free PMC article.
-
Cryo-electron microscopy and image classification reveal the existence and structure of the coxsackievirus A6 virion.Commun Biol. 2022 Sep 2;5(1):898. doi: 10.1038/s42003-022-03863-2. Commun Biol. 2022. PMID: 36056184 Free PMC article.
-
Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease.Expert Rev Vaccines. 2018 Sep;17(9):819-831. doi: 10.1080/14760584.2018.1510326. Epub 2018 Aug 22. Expert Rev Vaccines. 2018. PMID: 30095317 Review.
-
Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems.Expert Rev Anti Infect Ther. 2019 Apr;17(4):233-242. doi: 10.1080/14787210.2019.1585242. Epub 2019 Mar 6. Expert Rev Anti Infect Ther. 2019. PMID: 30793637 Review.
Cited by
-
A one-step reverse-transcription recombinase aided PCR assay for the rapid and sensitive detection of human enteroviruses.Biosaf Health. 2023 Mar 11;5(2):126-131. doi: 10.1016/j.bsheal.2023.03.002. eCollection 2023 Apr. Biosaf Health. 2023. PMID: 40078830 Free PMC article.
-
Completely conserved VP2 residue K140 of KREMEN1-dependent enteroviruses is critical for virus-receptor interactions and viral infection.mBio. 2025 Feb 5;16(2):e0304024. doi: 10.1128/mbio.03040-24. Epub 2025 Jan 16. mBio. 2025. PMID: 39817751 Free PMC article.
-
Molecular basis of Coxsackievirus A10 entry using the two-in-one attachment and uncoating receptor KRM1.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18711-18718. doi: 10.1073/pnas.2005341117. Epub 2020 Jul 20. Proc Natl Acad Sci U S A. 2020. PMID: 32690697 Free PMC article.
-
Characterization of Plaque Variants and the Involvement of Quasi-Species in a Population of EV-A71.Viruses. 2020 Jun 17;12(6):651. doi: 10.3390/v12060651. Viruses. 2020. PMID: 32560288 Free PMC article.
-
VP2 residue N142 of coxsackievirus A10 is critical for the interaction with KREMEN1 receptor and neutralizing antibodies and the pathogenicity in mice.PLoS Pathog. 2023 Oct 3;19(10):e1011662. doi: 10.1371/journal.ppat.1011662. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37788227 Free PMC article.
References
-
- Yamashita T, Ito M, Taniguchi A, Sakae K. Prevalence of coxsackievirus A5, A6, and A10 in patients with herpangina in Aichi Prefecture, 2005. Jpn. J. Infect. Dis. 2005;58:390–391. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

