Glyceraldehyde-3-phosphate dehydrogenase from Citrobacter sp. S-77 is post-translationally modified by CoA (protein CoAlation) under oxidative stress
- PMID: 30652074
- PMCID: PMC6325607
- DOI: 10.1002/2211-5463.12542
Glyceraldehyde-3-phosphate dehydrogenase from Citrobacter sp. S-77 is post-translationally modified by CoA (protein CoAlation) under oxidative stress
Abstract
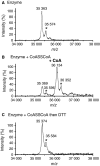
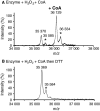
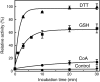
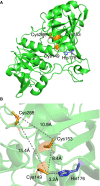
Protein CoAlation (S-thiolation by coenzyme A) has recently emerged as an alternative redox-regulated post-translational modification by which protein thiols are covalently modified with coenzyme A (CoA). However, little is known about the role and mechanism of this post-translational modification. In the present study, we investigated CoAlation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from a facultative anaerobic Gram-negative bacterium Citrobacter sp. S-77 (Cb GAPDH). GAPDH is a key glycolytic enzyme whose activity relies on the thiol-based redox-regulated post-translational modifications of active-site cysteine. LC-MS/MS analysis revealed that CoAlation of Cb GAPDH occurred in vivo under sodium hypochlorite (NaOCl) stress. The purified Cb GAPDH was highly sensitive to overoxidation by H2O2 and NaOCl, which resulted in irreversible enzyme inactivation. By contrast, treatment with coenzyme A disulphide (CoASSCoA) or H2O2/NaOCl in the presence of CoA led to CoAlation and inactivation of the enzyme; activity could be recovered after incubation with dithiothreitol, glutathione and CoA. CoAlation of the enzyme in vitro was confirmed by mass spectrometry. The presence of a substrate, glyceraldehyde-3-phosphate, fully protected Cb GAPDH from inactivation by CoAlation, suggesting that the inactivation is due to the formation of mixed disulphides between CoA and the active-site cysteine Cys149. A molecular docking study also supported the formation of mixed disulphide without steric constraints. These observations suggest that CoAlation is an alternative mechanism to protect the redox-sensitive thiol (Cys149) of Cb GAPDH against irreversible oxidation, thereby regulating enzyme activity under oxidative stress.
Keywords: S‐thiolation; coenzyme A; glyceraldehyde‐3‐phosphate dehydrogenase; post‐translational modification; redox regulation.
Figures







Similar articles
-
The glyceraldehyde-3-phosphate dehydrogenase GapDH of Corynebacterium diphtheriae is redox-controlled by protein S-mycothiolation under oxidative stress.Sci Rep. 2017 Jul 10;7(1):5020. doi: 10.1038/s41598-017-05206-2. Sci Rep. 2017. PMID: 28694441 Free PMC article.
-
Coenzyme A, protein CoAlation and redox regulation in mammalian cells.Biochem Soc Trans. 2018 Jun 19;46(3):721-728. doi: 10.1042/BST20170506. Epub 2018 May 25. Biochem Soc Trans. 2018. PMID: 29802218 Free PMC article. Review.
-
The thioredoxin-independent isoform of chloroplastic glyceraldehyde-3-phosphate dehydrogenase is selectively regulated by glutathionylation.FEBS J. 2007 Jan;274(1):212-26. doi: 10.1111/j.1742-4658.2006.05577.x. Epub 2006 Nov 28. FEBS J. 2007. PMID: 17140414
-
S-glutathionylation of glyceraldehyde-3-phosphate dehydrogenase induces formation of C150-C154 intrasubunit disulfide bond in the active site of the enzyme.Biochim Biophys Acta Gen Subj. 2017 Dec;1861(12):3167-3177. doi: 10.1016/j.bbagen.2017.09.008. Epub 2017 Sep 19. Biochim Biophys Acta Gen Subj. 2017. PMID: 28935607
-
[The biological significance of oxidative modifications of cysteine residues in proteins illustrated with the example of glyceraldehyde-3-phosphate dehydrogenase].Postepy Hig Med Dosw (Online). 2014 Mar 12;68:280-90. doi: 10.5604/17322693.1093929. Postepy Hig Med Dosw (Online). 2014. PMID: 24662796 Review. Polish.
Cited by
-
A Unique Mode of Coenzyme A Binding to the Nucleotide Binding Pocket of Human Metastasis Suppressor NME1.Int J Mol Sci. 2023 May 27;24(11):9359. doi: 10.3390/ijms24119359. Int J Mol Sci. 2023. PMID: 37298313 Free PMC article.
-
Profiling the Site of Protein CoAlation and Coenzyme A Stabilization Interactions.Antioxidants (Basel). 2022 Jul 14;11(7):1362. doi: 10.3390/antiox11071362. Antioxidants (Basel). 2022. PMID: 35883853 Free PMC article.
-
Functional interplay among thiol-based redox signaling, metabolism, and ferroptosis unveiled by a genetic variant of TP53.Proc Natl Acad Sci U S A. 2020 Oct 27;117(43):26804-26811. doi: 10.1073/pnas.2009943117. Epub 2020 Oct 14. Proc Natl Acad Sci U S A. 2020. PMID: 33055209 Free PMC article.
-
The Writers, Readers, and Erasers in Redox Regulation of GAPDH.Antioxidants (Basel). 2020 Dec 16;9(12):1288. doi: 10.3390/antiox9121288. Antioxidants (Basel). 2020. PMID: 33339386 Free PMC article. Review.
-
Bacillus subtilis YtpP and Thioredoxin A Are New Players in the Coenzyme-A-Mediated Defense Mechanism against Cellular Stress.Antioxidants (Basel). 2023 Apr 15;12(4):938. doi: 10.3390/antiox12040938. Antioxidants (Basel). 2023. PMID: 37107313 Free PMC article.
References
-
- Antelmann H and Hamilton CJ (2012) Bacterial mechanisms of reversible protein S‐thiolation: structural and mechanistic insights into mycoredoxins. Mol Microbiol 86, 759–764. - PubMed
-
- Van Laer K, Hamilton CJ and Messens J (2013) Low‐molecular‐weight thiols in thiol‐disulfide exchange. Antioxid Redox Signal 18, 1642–1653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

