Uncommon properties of lipid biosynthesis of isolated plastids in the unicellular red alga Cyanidioschyzon merolae
- PMID: 30652079
- PMCID: PMC6325583
- DOI: 10.1002/2211-5463.12551
Uncommon properties of lipid biosynthesis of isolated plastids in the unicellular red alga Cyanidioschyzon merolae
Abstract
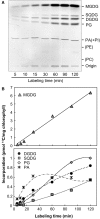
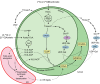
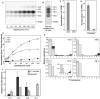
Red algae are a large group of photosynthetic eukaryotes that diverged from green algae over one billion years ago, and have various traits distinct from those of both green algae and land plants. Although most red algae are marine species (both unicellular and macrophytic), the Cyanidiales class of red algae includes unicellular species which live in hot springs, such as Cyanidioschyzon merolae, which is a model species for biochemical and molecular biological studies. Lipid metabolism in red algae has previously been studied in intact cells. Here, we present the results of radiolabeling and stable isotope labeling experiments in intact plastids isolated from the unicellular red alga C. merolae. We focused on two uncommon features: First, the galactose moiety of monogalactosyldiacylglycerol was efficiently labeled with bicarbonate, indicating that an unknown pathway for providing UDP-galactose exists within the plastid. Second, saturated fatty acids, namely, palmitic and stearic acids, were the sole products of fatty acid synthesis in the plastid, and they were efficiently exported. This finding suggests that the endoplasmic reticulum is the sole site of desaturation. We present a general principle of red algal lipid biosynthesis, namely, 'indigenous C18 fatty acids are neither desaturated nor directly utilized within the plastid'. We believe that this is valid in both C. merolae lacking polyunsaturated fatty acids and marine red algae with a high content of arachidonic and eicosapentaenoic acids.
Keywords: Cyanidioschyzon merolae; isolated plastids; radiolabeling experiment; red alga; stable isotope; stearoyl‐ACP desaturase.
Figures





References
-
- Sato N, Moriyama T, Mori N and Toyoshima M (2017) Lipid metabolism and potentials of biofuel and high added‐value oil production in red algae. World J Microbiol Biotechnol 33, 74. - PubMed
-
- Kuroiwa T, Miyagishima S, Matsunaga S, Sato N, Nozaki H, Tanaka K and Misumi O (eds) (2018) Cyanidioschyzon merolae. A New Model Eukaryote for Cell and Organelle Biology. Springer, Singapore.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

