Coprs inactivation leads to a derepression of LINE1 transposons in spermatocytes
- PMID: 30652083
- PMCID: PMC6325579
- DOI: 10.1002/2211-5463.12562
Coprs inactivation leads to a derepression of LINE1 transposons in spermatocytes
Erratum in
-
Erratum to: Coprs inactivation leads to a derepression of LINE1 transposons in spermatocytes.FEBS Open Bio. 2019 Feb 28;9(3):558. doi: 10.1002/2211-5463.12607. eCollection 2019 Mar. FEBS Open Bio. 2019. PMID: 30868064 Free PMC article.
Abstract
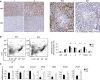
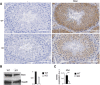
Repression of retrotransposons is essential for genome integrity during germ cell development and is tightly controlled through epigenetic mechanisms. In primordial germ cells, protein arginine N-methyltransferase (Prmt5) is involved in retrotransposon repression by methylating Piwi proteins, which is part of the piRNA pathway. Here, we show that in mice, genetic inactivation of coprs (which is highly expressed in testis and encodes a histone-binding protein required for the targeting of Prmt5 activity) affects the maturation of spermatogonia to spermatids. Mass spectrometry analysis revealed the presence of Miwi in testis protein lysates immunoprecipitated with an anti-Coprs antibody. The observed deregulation of Miwi and pachytene pre-piRNAs levels and the derepression of LINE1 repetitive sequences observed in coprs-/- mice suggest that Coprs is implicated in genome surveillance mechanisms.
Keywords: Miwi; coprs; piRNA; spermatocyte; teratozoospermia; testis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Fang J, Feng Q, Ketel CS, Wang H, Cao R, Xia L, Erdjument‐Bromage H, Tempst P, Simon JA and Zhang Y (2002) Purification and functional characterization of SET8, a nucleosomal histone H4‐lysine 20‐specific methyltransferase. Curr Biol 12, 1086–1099. - PubMed
-
- Kimmins S and Sassone‐Corsi P (2005) Chromatin remodelling and epigenetic features of germ cells. Nature 434, 583–589. - PubMed
-
- Lacham‐Kaplan O (2004) In vivo and in vitro differentiation of male germ cells in the mouse. Reproduction 128, 147–152. - PubMed
-
- McLaren A (2000) Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol 163, 3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

