Using simulation studies to evaluate statistical methods
- PMID: 30652356
- PMCID: PMC6492164
- DOI: 10.1002/sim.8086
Using simulation studies to evaluate statistical methods
Abstract
Simulation studies are computer experiments that involve creating data by pseudo-random sampling. A key strength of simulation studies is the ability to understand the behavior of statistical methods because some "truth" (usually some parameter/s of interest) is known from the process of generating the data. This allows us to consider properties of methods, such as bias. While widely used, simulation studies are often poorly designed, analyzed, and reported. This tutorial outlines the rationale for using simulation studies and offers guidance for design, execution, analysis, reporting, and presentation. In particular, this tutorial provides a structured approach for planning and reporting simulation studies, which involves defining aims, data-generating mechanisms, estimands, methods, and performance measures ("ADEMP"); coherent terminology for simulation studies; guidance on coding simulation studies; a critical discussion of key performance measures and their estimation; guidance on structuring tabular and graphical presentation of results; and new graphical presentations. With a view to describing recent practice, we review 100 articles taken from Volume 34 of Statistics in Medicine, which included at least one simulation study and identify areas for improvement.
Keywords: Monte Carlo; graphics for simulation; simulation design; simulation reporting; simulation studies.
© 2019 The Authors. Statistics in Medicine Published by John Wiley & Sons Ltd.
Figures

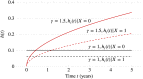

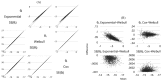





References
-
- Feiveson AH. Power by simulation. Stata J. 2002;2(2):107‐124.
-
- Rubin DB. Bayesianly justifiable and relevant frequency calculations for the applies statistician. Ann Stat. 1984;12(4):1151‐1172.
-
- Grieve AP. Idle thoughts of a ‘well‐calibrated’ Bayesian in clinical drug development. Pharm Stat. 2016;15(2):96‐108. - PubMed
-
- Hoaglin DC, Andrews DF. The reporting of computation‐based results in statistics. Am Stat. 1975;29(3):122‐126.
-
- Hauck WW, Anderson S. A survey regarding the reporting of simulation studies. Am Stat. 1984;38(3):214‐216.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
