Gender Difference in Damage-Mediated Signaling Contributes to Pulmonary Arterial Hypertension
- PMID: 30652485
- PMCID: PMC6765065
- DOI: 10.1089/ars.2018.7664
Gender Difference in Damage-Mediated Signaling Contributes to Pulmonary Arterial Hypertension
Abstract
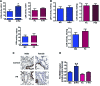
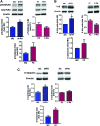
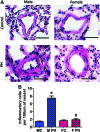
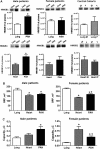
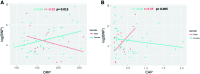
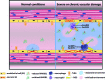
Aims: Pulmonary arterial hypertension (PAH) is a progressive lethal disease with a known gender dimorphism. Female patients are more susceptible to PAH, whereas male patients have a lower survival rate. Initial pulmonary vascular damage plays an important role in PAH pathogenesis. Therefore, this study aimed at investigating the role of gender in activation of apoptosis/necrosis-mediated signaling pathways in PAH. Results: The media collected from pulmonary artery endothelial cells (PAECs) that died by necrosis or apoptosis were used to treat naive PAECs. Necrotic cell death stimulated phosphorylation of toll-like receptor 4, accumulation of interleukin 1 beta, and expression of E-selectin in a redox-dependent manner; apoptosis did not induce any of these effects. In the animal model of severe PAH, the necrotic marker, high mobility group box 1 (HMGB1), was visualized in the pulmonary vascular wall of male but not female rats. This vascular necrosis was associated with male-specific redox changes in plasma, activation of the same inflammatory signaling pathway seen in response to necrosis in vitro, and an increased endothelial-leukocyte adhesion in small pulmonary arteries. In PAH patients, gender-specific changes in redox homeostasis correlated with the prognostic marker, B-type natriuretic peptide. Males had also shown elevated circulating levels of HMGB1 and pro-inflammatory changes. Innovation: This study discovered the role of gender in the initiation of damage-associated signaling in PAH and highlights the importance of the gender-specific approach in PAH therapy. Conclusion: In PAH, the necrotic cell death is augmented in male patients compared with female patients. Factors released from necrotic cells could alter redox homeostasis and stimulate inflammatory signaling pathways.
Keywords: gender difference; inflammation; necrosis; pulmonary hypertension.
Conflict of interest statement
No competing financial interests exist.
Figures

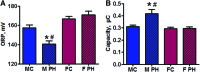

Similar articles
-
Necrosis-Released HMGB1 (High Mobility Group Box 1) in the Progressive Pulmonary Arterial Hypertension Associated With Male Sex.Hypertension. 2020 Dec;76(6):1787-1799. doi: 10.1161/HYPERTENSIONAHA.120.16118. Epub 2020 Oct 5. Hypertension. 2020. PMID: 33012199 Free PMC article.
-
HMGB1/TLR4 promotes hypoxic pulmonary hypertension via suppressing BMPR2 signaling.Vascul Pharmacol. 2019 Jun;117:35-44. doi: 10.1016/j.vph.2018.12.006. Epub 2019 Jan 3. Vascul Pharmacol. 2019. PMID: 30610955
-
ERK/Drp1-dependent mitochondrial fission contributes to HMGB1-induced autophagy in pulmonary arterial hypertension.Cell Prolif. 2021 Jun;54(6):e13048. doi: 10.1111/cpr.13048. Epub 2021 May 4. Cell Prolif. 2021. PMID: 33948998 Free PMC article.
-
[Role of high-mobility group B1 in pulmonary arterial hypertension].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020 May;32(5):625-631. doi: 10.3760/cma.j.cn121430-20200106-00107. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020. PMID: 32576360 Review. Chinese.
-
Focus on Early Events: Pathogenesis of Pulmonary Arterial Hypertension Development.Antioxid Redox Signal. 2019 Nov 1;31(13):933-953. doi: 10.1089/ars.2018.7673. Epub 2019 Jul 2. Antioxid Redox Signal. 2019. PMID: 31169021 Free PMC article. Review.
Cited by
-
Sex-specific stress response and HMGB1 release in pulmonary endothelial cells.PLoS One. 2020 Apr 9;15(4):e0231267. doi: 10.1371/journal.pone.0231267. eCollection 2020. PLoS One. 2020. PMID: 32271800 Free PMC article.
-
Necrosis Contributes to the Development of Hypertension in Male, but Not Female, Spontaneously Hypertensive Rats.Hypertension. 2019 Dec;74(6):1524-1531. doi: 10.1161/HYPERTENSIONAHA.119.13477. Epub 2019 Oct 28. Hypertension. 2019. PMID: 31656095 Free PMC article.
-
The Role of HMGB1, a Nuclear Damage-Associated Molecular Pattern Molecule, in the Pathogenesis of Lung Diseases.Antioxid Redox Signal. 2019 Nov 1;31(13):954-993. doi: 10.1089/ars.2019.7818. Epub 2019 Jul 11. Antioxid Redox Signal. 2019. PMID: 31184204 Free PMC article. Review.
-
Mechanisms of lung endothelial cell injury and survival in pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2024 Dec 1;327(6):L972-L983. doi: 10.1152/ajplung.00208.2024. Epub 2024 Oct 15. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39406383 Free PMC article. Review.
-
Prenatal Sildenafil and Fetal-placental Programming in Human Pregnancies Complicated by Fetal Growth Restriction: A Retrospective Gene Expression Analysis.J Trial Error. 2023 Sep 26:e16. doi: 10.36850/e16. Online ahead of print. J Trial Error. 2023. PMID: 39404670 Free PMC article.
References
-
- Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, and Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010 - PubMed
-
- Amabile N, Heiss C, Chang V, Angeli FS, Damon L, Rame EJ, McGlothlin D, Grossman W, De Marco T, and Yeghiazarians Y. Increased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J Heart Lung Transplant 28: 1081–1086, 2009 - PubMed
-
- Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, and Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 103: 1245–1256, 2009 - PubMed
-
- Basu S, Binder RJ, Suto R, Anderson KM, and Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12: 1539–1546, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

