Metal bashing: iron deficiency and manganese overexposure impact on peripheral nerves
- PMID: 30652531
- PMCID: PMC6397089
- DOI: 10.1080/15287394.2019.1566105
Metal bashing: iron deficiency and manganese overexposure impact on peripheral nerves
Abstract
Iron (Fe) deficiency (FeD) and manganese (Mn) overexposure (MnOE) may result in several neurological alterations in the nervous system. Iron deficiency produces unique neurological deficits due to its elemental role in central nervous system (CNS) development and myelination, which might persist after normalization of Fe in the diet. Conversely, MnOE is associated with diverse neurocognitive deficits. Despite these well-known neurotoxic effects on the CNS, the influence of FeD and MnOE on the peripheral nervous system (PNS) remains poorly understood. The aim of the present investigation was to examine the effects of developmental FeD and MnOE or their combination on the sciatic nerve of young and adult rats. The parameters measured included divalent metal transporter 1 (DMT1), transferrin receptor (TfR), myelin basic protein (MBP) and peripheral myelin protein 22 (PMP22) expression, as well as Fe levels in the nerve. Our results showed that FeD produced a significant reduction in MBP and PMP22 content at P29, which persisted at P60 after Fe-sufficient diet replenishment regardless of Mn exposure levels. At P60 MnOE significantly increased sciatic nerve Fe content and DMT1 expression. However, the combination of FeD and MnOE produced no marked motor skill impairment. Evidence indicates that FeD appears to hinder developmental peripheral myelination, while MnOE may directly alter Fe homeostasis. Further studies are required to elucidate the interplay between these pathological conditions.
Keywords: Iron; manganese; myelination; peripheral nervous system.
Conflict of interest statement
Figures
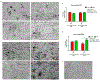
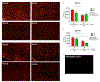
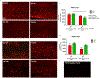
Similar articles
-
Effects of developmental exposure to manganese and/or low iron diet: Changes to metal transporters, sucrose preference, elevated zero-maze, open-field, and locomotion in response to fenfluramine, amphetamine, and MK-801.Toxicol Rep. 2015;2:1046-1056. doi: 10.1016/j.toxrep.2015.07.015. Toxicol Rep. 2015. PMID: 26295019 Free PMC article.
-
Developmental manganese neurotoxicity in rats: Cognitive deficits in allocentric and egocentric learning and memory.Neurotoxicol Teratol. 2017 Jan-Feb;59:16-26. doi: 10.1016/j.ntt.2016.10.005. Epub 2016 Oct 15. Neurotoxicol Teratol. 2017. PMID: 27756629 Free PMC article.
-
Developmental manganese exposure in combination with developmental stress and iron deficiency: Effects on behavior and monoamines.Neurotoxicol Teratol. 2016 Jul-Aug;56:55-67. doi: 10.1016/j.ntt.2016.06.004. Epub 2016 Jun 11. Neurotoxicol Teratol. 2016. PMID: 27302314 Free PMC article.
-
Interactions between iron and manganese in neurotoxicity.Arch Toxicol. 2020 Mar;94(3):725-734. doi: 10.1007/s00204-020-02652-2. Epub 2020 Mar 16. Arch Toxicol. 2020. PMID: 32180038 Review.
-
Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency.J Trace Elem Med Biol. 2017 May;41:41-53. doi: 10.1016/j.jtemb.2017.02.005. Epub 2017 Feb 12. J Trace Elem Med Biol. 2017. PMID: 28347462 Review.
Cited by
-
The Role of Dietary Nutrients in Peripheral Nerve Regeneration.Int J Mol Sci. 2021 Jul 10;22(14):7417. doi: 10.3390/ijms22147417. Int J Mol Sci. 2021. PMID: 34299037 Free PMC article. Review.
-
Elemental Imbalances After Manganese Exposure and the Regulatory Potential of Curcumin.Biol Trace Elem Res. 2025 Mar 25. doi: 10.1007/s12011-025-04586-1. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40133721
References
-
- Akyol A, Kiylioglu N, Kadikoylu G, Bolaman AZ and Ozgel N 2003. Iron deficiency anemia and restless legs syndrome: Is there an electrophysiological abnormality? Clin Neurol Neurosurg 106: 23–27. - PubMed
-
- Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B 2003. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res 53:217–223 - PubMed
-
- Amos-Kroohs R:M, Bloor CP, Qureshi MA, Vorhees CV and Williams MT 2015. Effects of developmental exposure to manganese and/or low iron diet: Changes to metal transporters, sucrose preference, elevated zero-maze, open-field, and locomotion in response to fenfluramine, amphetamine, and MK-801. Toxicol Rep 2: 1046–1056. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
