Using a Federated Network of Real-World Data to Optimize Clinical Trials Operations
- PMID: 30652541
- PMCID: PMC6816049
- DOI: 10.1200/CCI.17.00067
Using a Federated Network of Real-World Data to Optimize Clinical Trials Operations
Abstract
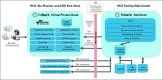
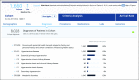
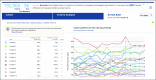
Clinical trials, whether industry, cooperative group sponsored, or investigator initiated, have an unacceptable rate of failure as a result of the inability to recruit sufficient numbers of patients. Even those trials that are completed often require time-consuming protocol amendments to achieve accrual goals. These inefficiencies in clinical trial research result in increasing costs and prolong the time needed to bring improved treatments to cancer clinical practice. TriNetX has developed a clinical research collaboration platform-deployed by a federated network of health care organizations (HCOs), pharmaceutical firms (Pharma), and contract research organizations (CROs)-to enable data-driven clinical research study design to reduce accrual failure and protocol amendment. Currently, the network extends to 55 HCOs and covers 84 million patients, mostly within the United States, but with a growing international presence. (Many of the HCOs in United States are Clinical and Translational Science Awardees and/or National Cancer Institute-designated cancer centers.) The TriNetX business model includes Pharma and the CROs as sponsors whose subscriptions financially support the network, including the software and hardware costs of the HCOs. Furthermore, as each HCO network member has their data harmonized with the TriNetX model upon joining, data sharing among them does not require any technical processes to establish connectivity. To date, on the basis of the data on the network, HCOs have been presented approximately 757 studies by Pharma and CROs, and four data-sharing subnetworks have been formed among member HCOs.
Conflict of interest statement
Umit Topaloglu
No relationship to disclose
Matvey Palchuk
Figures
References
-
- Topaloglu U, Niedner H: Advances in clinical research towards the data-driven economy. Clin Res 30:58-62, 2016
-
- Heinze D, Kahn S, McOwen P, et al. : Building a richly connected and highly analyzed genotype/phenotype ecosystem in a world of data silos. 2014 AMIA Joint Summits on Translational Science, San Francisco, CA, April 7-11, 2014
-
- US Food and Drug Administration : Guidance for industry: Use of electronic health record data in clinical investigations. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat...
-
- Rosenbaum L: Bridging the data-sharing divide: Seeing the devil in the details, not the other camp. N Engl J Med 376:2201-2203, 2017 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

