What is the appropriate duration of adjuvant imatinib mesylate treatment for primary gastrointestinal stromal tumors classified according to the strict definition of tumor rupture?
- PMID: 30653164
- PMCID: PMC6370173
- DOI: 10.1097/MD.0000000000014177
What is the appropriate duration of adjuvant imatinib mesylate treatment for primary gastrointestinal stromal tumors classified according to the strict definition of tumor rupture?
Abstract
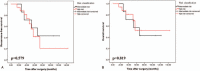
In gastrointestinal stromal tumors (GISTs), rupture is a high-risk feature; however, "tumor rupture" is inconsistently defined, and its prognostic value remains controversial.Six hundred ninety-one patients undergoing surgery for primary nonmetastatic GISTs from 2003 to 2015 at our institution were enrolled. The strict definitions of "tumor rupture" according to the Kinki GIST Study Group (KGSG) were used.The median follow-up time was 64 months. The 5-year recurrence-free survival (RFS) and overall survival (OS) rates in the entire group were 79.3% and 84.1%, respectively. According to the KGSG's definition, tumor rupture occurred only in 24 (3.5%) of 691 patients. For all 691 patients, multivariable analysis showed that tumor rupture, according to KGSG's definition, is one of the independently prognostic factors for both RFS and OS. Twenty-four patients with tumor rupture were further analyzed. Receiving IM for more than 3 years was significantly associated with improved RFS and OS in GISTs patients with tumor rupture.Tumor rupture according to KGSG's definition was an independent predictive factor associated with GIST patient prognosis. More importantly, for GISTs with tumor rupture according to the KGSG's strict definition, receiving IM treatment for ≥3 years should be considered.
Conflict of interest statement
The authors have no conflicts of interest to disclose
Figures


Similar articles
-
Recurrence-Free Survival After Resection of Gastric Gastrointestinal Stromal Tumors Classified According to a Strict Definition of Tumor Rupture: A Population-Based Study.Ann Surg Oncol. 2018 May;25(5):1133-1139. doi: 10.1245/s10434-018-6353-5. Epub 2018 Feb 12. Ann Surg Oncol. 2018. PMID: 29435684
-
Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients With High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up.JAMA Oncol. 2020 Aug 1;6(8):1241-1246. doi: 10.1001/jamaoncol.2020.2091. JAMA Oncol. 2020. PMID: 32469385 Free PMC article. Clinical Trial.
-
Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial.JAMA Oncol. 2017 May 1;3(5):602-609. doi: 10.1001/jamaoncol.2016.5751. JAMA Oncol. 2017. PMID: 28334365 Free PMC article. Clinical Trial.
-
Management of localized gastrointestinal stromal tumors and adjuvant therapy with imatinib.Anticancer Drugs. 2012 Jun;23 Suppl:S3-6. doi: 10.1097/CAD.0b013e3283559fab. Anticancer Drugs. 2012. PMID: 22739667 Review.
-
Preoperative imatinib treatment in patients with advanced gastrointestinal stromal tumors: patient experiences and systematic review of 563 patients.Int Surg. 2015 May;100(5):860-9. doi: 10.9738/INTSURG-D-14-00178.1. Int Surg. 2015. PMID: 26011207 Free PMC article.
Cited by
-
Rectovaginal extragastrointestinal stromal tumour (EGIST): an additional entity to be considered in the differential diagnosis of tumours of the rectovaginal septum.BMJ Case Rep. 2021 Mar 8;14(3):e237669. doi: 10.1136/bcr-2020-237669. BMJ Case Rep. 2021. PMID: 33685909 Free PMC article. Review.
-
Gastrointestinal stromal tumor presenting as a rectovaginal septal mass: A case report and review of literature.Medicine (Baltimore). 2019 Apr;98(17):e15398. doi: 10.1097/MD.0000000000015398. Medicine (Baltimore). 2019. PMID: 31027138 Free PMC article. Review.
-
Management of subepithelial esophageal tumors.Innov Surg Sci. 2024 Aug 20;10(1):21-30. doi: 10.1515/iss-2023-0011. eCollection 2025 Mar. Innov Surg Sci. 2024. PMID: 40144787 Free PMC article. Review.
-
Re-appraisal of the universal definition of tumor rupture among patients with high-risk gastrointestinal stromal tumors.Ann Gastroenterol Surg. 2023 Apr 26;7(6):1021-1031. doi: 10.1002/ags3.12684. eCollection 2023 Nov. Ann Gastroenterol Surg. 2023. PMID: 37927930 Free PMC article.
-
Survival of patients with ruptured gastrointestinal stromal tumour treated with adjuvant imatinib in a randomised trial.Br J Cancer. 2024 Jul;131(2):299-304. doi: 10.1038/s41416-024-02738-z. Epub 2024 Jun 11. Br J Cancer. 2024. PMID: 38862742 Free PMC article. Clinical Trial.
References
-
- Yanagimoto Y, Takahashi T, Muguruma K, et al. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer 2015;2:426–33. - PubMed
-
- Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 2010;12:1854–9. - PubMed
-
- Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;3:265–74. - PubMed
-
- Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour—the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 2011;10:890–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

