Prevalence of Hepatitis B Virus, Hepatitis C Virus, and HIV Infection Among Patients With Newly Diagnosed Cancer From Academic and Community Oncology Practices
- PMID: 30653226
- PMCID: PMC6459217
- DOI: 10.1001/jamaoncol.2018.6437
Prevalence of Hepatitis B Virus, Hepatitis C Virus, and HIV Infection Among Patients With Newly Diagnosed Cancer From Academic and Community Oncology Practices
Erratum in
-
Errors in Author Affiliations and Additional Contributions.JAMA Oncol. 2019 Apr 1;5(4):579. doi: 10.1001/jamaoncol.2019.0220. JAMA Oncol. 2019. PMID: 30844022 Free PMC article. No abstract available.
Abstract
Importance: Universal screening of patients with newly diagnosed cancer for hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV is not routine in oncology practice, and experts disagree about whether universal screening should be performed.
Objective: To estimate the prevalence of HBV, HCV, and HIV infection among persons with newly diagnosed cancer.
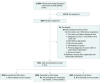
Design, setting, and participants: Multicenter prospective cohort study of patients with newly diagnosed cancer (ie, identified within 120 days of cancer diagnosis) at 9 academic and 9 community oncology institutions affiliated with SWOG (formerly the Southwest Oncology Group) Cancer Research Network, a member of the National Clinical Trials Network, with enrollment from August 29, 2013, through February 15, 2017. The data analysis was conducted using data available through August 17, 2017.
Main outcomes and measures: The accrual goal was 3000 patients and the primary end point was the presence of HBV infection (previous or chronic), HCV infection, or HIV infection at enrollment. Patients with previous knowledge of infection as well as patients with unknown viral viral status were evaluated.
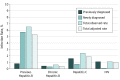
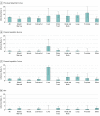
Results: Of 3092 registered patients, 3051 were eligible and evaluable. Median (range) age was 60.6 (18.2-93.7) years, 1842 (60.4%) were female, 553 (18.1%) were black, and 558 (18.3%) were Hispanic ethnicity. Screened patients had similar clinical and demographic characteristics compared with those registered. The observed infection rate for previous HBV infection was 6.5% (95% CI, 5.6%-7.4%; n = 197 of 3050 patients); chronic HBV, 0.6% (95% CI, 0.4%-1.0%; n = 19 of 3050 patients); HCV, 2.4% (95% CI, 1.9%-3.0%; n = 71 of 2990 patients); and HIV, 1.1% (95% CI, 0.8%-1.6%; n = 34 of 3045). Among those with viral infections, 8 patients with chronic HBV (42.1%; 95% CI, 20.3%-66.5%), 22 patients with HCV (31.0%; 95% CI, 20.5%-43.1%), and 2 patients with HIV (5.9%; 95% CI, 0.7%-19.7%) were newly diagnosed through the study. Among patients with infections, 4 patients with chronic HBV (21.1%; 95% CI, 6.1%-45.6%), 23 patients with HCV (32.4%; 95% CI, 21.8%-44.5%), and 7 patients with HIV (20.6%; 95% CI, 8.7%-37.9%) had no identifiable risk factors.
Conclusions and relevance: Results of this study found that a substantial proportion of patients with newly diagnosed cancer and concurrent HBV or HCV are unaware of their viral infection at the time of cancer diagnosis, and many had no identifiable risk factors for infection. Screening patients with cancer to identify HBV and HCV infection before starting treatment may be warranted to prevent viral reactivation and adverse clinical outcomes. The low rate of undiagnosed HIV infection may not support universal screening of newly diagnosed cancer patients.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention. National health interview survey. https://www.cdc.gov/nchs/nhis/index.htm. Accessed March 1, 2018.
-
- Centers for Disease Control and Prevention. Hepatitis risk assessment. https://www.cdc.gov/hepatitis/riskassessment/index.htm. Accessed March 1, 2018.
-
- Centers for Disease Control and Prevention HIV 5 surveillance report, 2016. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveil.... Accessed August 20, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

