Discovery of a Series of 2'-α-Fluoro,2'-β-bromo-ribonucleosides and Their Phosphoramidate Prodrugs as Potent Pan-Genotypic Inhibitors of Hepatitis C Virus
- PMID: 30653317
- PMCID: PMC7722249
- DOI: 10.1021/acs.jmedchem.8b01300
Discovery of a Series of 2'-α-Fluoro,2'-β-bromo-ribonucleosides and Their Phosphoramidate Prodrugs as Potent Pan-Genotypic Inhibitors of Hepatitis C Virus
Abstract
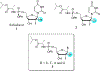
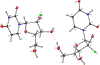
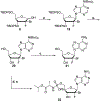
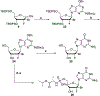
Hepatitis C virus (HCV) nucleoside inhibitors display pan-genotypic activity, a high barrier to the selection of resistant virus, and are some of the most potent direct-acting agents with durable sustained virologic response in humans. Herein, we report, the discovery of β-d-2'-Br,2'-F-uridine phosphoramidate diastereomers 27 and 28, as nontoxic pan-genotypic anti-HCV agents. Extensive profiling of these two phosphorous diastereomers was performed to select one for in-depth preclinical profiling. The 5'-triphosphate formed from these phosphoramidates selectively inhibited HCV NS5B polymerase with no inhibition of human polymerases and cellular mitochondrial RNA polymerase up to 100 μM. Both are nontoxic by a variety of measures and display good stability in human blood and favorable metabolism in human intestinal microsomes and liver microsomes. Ultimately, a preliminary oral pharmacokinetics study in male beagles showed that 28 is superior to 27 and is an attractive candidate for further studies to establish its potential value as a new clinical anti-HCV agent.
Figures
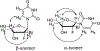


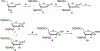
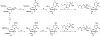
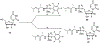
References
-
- Hepatitis C, Global Health Report; World Health Organization, 2017. http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c (accessed July 10, 2018).
-
- Fried MW; Shiffman ML; Reddy KR; Smith C; Marinos G; Goncales FL; Haussinger D; Diango M; Carosi G; Dhumeaux D; Craxi A; Lin A; Hoffman J; Yu J Peginterferon Alfa-2a plus Ribavirin for Chronic Hepatitis C Virus Infection. N. Engl. J. Med 2002, 347, 975–982. - PubMed
-
- Bartenschlager R; Lohmann V; Penin F The Molecular and Structural Basis of Advanced Antiviral Therapy for Hepatitis C Virus Infection. Nat. Rev. Microbiol 2013, 11, 482–496. - PubMed
-
- Götte M; Feld JJ Direct-acting Antiviral Agents for Hepatitis C: Structural and Mechanistic Insights. Nat. Rev. Gastroenterol. Hepatol 2016, 13, 338–351. - PubMed
-
- Manns MP; Foster GR; Rockstroh JK; Zeuzem S; Zoulim F; Houghton M The way Forward in HCV Treatment - Finding the Right Path. Nat. Rev. Drug Discovery 2007, 6, 991–1000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

