Autologous Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: Results of the BMT CTN 0702 Trial
- PMID: 30653422
- PMCID: PMC6553842
- DOI: 10.1200/JCO.18.00685
Autologous Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: Results of the BMT CTN 0702 Trial
Abstract
Purpose: Single-cycle melphalan 200 mg/m2 and autologous hematopoietic cell transplantation (AHCT) followed by lenalidomide (len) maintenance have improved progression-free survival (PFS) and overall survival (OS) for transplantation-eligible patients with multiple myeloma (MM). We designed a prospective, randomized, phase III study to test additional interventions to improve PFS by comparing AHCT, tandem AHCT (AHCT/AHCT), and AHCT and four subsequent cycles of len, bortezomib, and dexamethasone (RVD; AHCT + RVD), all followed by len until disease progression.
Patients and methods: Patients with symptomatic MM within 12 months from starting therapy and without progression who were age 70 years or younger were randomly assigned to AHCT/AHCT + len (n = 247), AHCT + RVD + len (n = 254), or AHCT + len (n = 257). The primary end point was 38-month PFS.
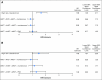
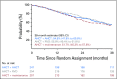
Results: The study population had a median age of 56 years (range, 20 to 70 years); 24% of patients had high-risk MM, 73% had a triple-drug regimen as initial therapy, and 18% were in complete response at enrollment. The 38-month PFS rate was 58.5% (95% CI, 51.7% to 64.6%) for AHCT/AHCT + len, 57.8% (95% CI, 51.4% to 63.7%) for AHCT + RVD + len, and 53.9% (95% CI, 47.4% to 60%) for AHCT + len. For AHCT/AHCT + len, AHCT + RVD + len, and AHCT + len, the OS rates were 81.8% (95% CI, 76.2% to 86.2%), 85.4% (95% CI, 80.4% to 89.3%), and 83.7% (95% CI, 78.4% to 87.8%), respectively, and the complete response rates at 1 year were 50.5% (n = 192), 58.4% (n = 209), and 47.1% (n = 208), respectively. Toxicity profiles and development of second primary malignancies were similar across treatment arms.
Conclusion: Second AHCT or RVD consolidation as post-AHCT interventions for the up-front treatment of transplantation-eligible patients with MM did not improve PFS or OS. Single AHCT and len should remain as the standard approach for this population.
Trial registration: ClinicalTrials.gov NCT01109004.
Conflict of interest statement
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding parties.
Figures


References
-
- Palumbo A, Triolo S, Argentino C, et al. : Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood 94:1248-1253, 1999 - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. : High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875-1883, 2003 - PubMed
-
- Attal M, Harousseau JL, Stoppa AM, et al. : A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma: Intergroupe Français du Myélome. N Engl J Med 335:91-97, 1996 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL069294/HL/NHLBI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- UG1 HL069286/HL/NHLBI NIH HHS/United States
- UG1 HL069290/HL/NHLBI NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- UG1 HL069315/HL/NHLBI NIH HHS/United States
- UG1 HL069278/HL/NHLBI NIH HHS/United States
- UG1 HL069246/HL/NHLBI NIH HHS/United States
- UG1 HL069274/HL/NHLBI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- U10 HL069274/HL/NHLBI NIH HHS/United States
- UG1 HL069249/HL/NHLBI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 HL109137/HL/NHLBI NIH HHS/United States

