The Role of Complement in Liver Injury, Regeneration, and Transplantation
- PMID: 30653682
- PMCID: PMC6771474
- DOI: 10.1002/hep.30508
The Role of Complement in Liver Injury, Regeneration, and Transplantation
Abstract
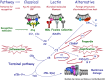
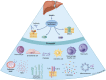
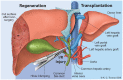
The liver is both an immunologically complex and a privileged organ. The innate immune system is a central player, in which the complement system emerges as a pivotal part of liver homeostasis, immune responses, and crosstalk with other effector systems in both innate and adaptive immunity. The liver produces the majority of the complement proteins and is the home of important immune cells such as Kupffer cells. Liver immune responses are delicately tuned between tolerance to many antigens flowing in from the alimentary tract, a tolerance that likely makes the liver less prone to rejection than other solid organ transplants, and reaction to local injury, systemic inflammation, and regeneration. Notably, complement is a double-edged sword as activation is detrimental by inducing inflammatory tissue damage in, for example, ischemia-reperfusion injury and transplant rejection yet is beneficial for liver tissue regeneration. Therapeutic complement inhibition is rapidly developing for routine clinical treatment of several diseases. In the liver, targeted inhibition of damaged tissue may be a rational and promising approach to avoid further tissue destruction and simultaneously preserve beneficial effects of complement in areas of proliferation. Here, we argue that complement is a key system to manipulate in the liver in several clinical settings, including liver injury and regeneration after major surgery and preservation of the organ during transplantation.
© 2019 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of American Association for the Study of Liver Diseases.
Figures
References
-
- Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol 2006;3:333‐340. - PubMed
-
- Mahmoudi M, Nilsson PH, Mollnes TE, Roos D, Sullivan KE. Complement deficiencies In: Rezaei N, Aghamohammadi A, Notarangelo L, eds. Primary Immunodeficiency Diseases: Definition, Diagnosis, and Management. 2nd edn Heidelberg: Springer; 2017:437‐460.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

