Hyperspectral imaging in automated digital dermoscopy screening for melanoma
- PMID: 30653684
- PMCID: PMC6519386
- DOI: 10.1002/lsm.23055
Hyperspectral imaging in automated digital dermoscopy screening for melanoma
Abstract
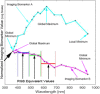
Objectives: Early melanoma detection decreases morbidity and mortality. Early detection classically involves dermoscopy to identify suspicious lesions for which biopsy is indicated. Biopsy and histological examination then diagnose benign nevi, atypical nevi, or cancerous growths. With current methods, a considerable number of unnecessary biopsies are performed as only 11% of all biopsied, suspicious lesions are actually melanomas. Thus, there is a need for more advanced noninvasive diagnostics to guide the decision of whether or not to biopsy. Artificial intelligence can generate screening algorithms that transform a set of imaging biomarkers into a risk score that can be used to classify a lesion as a melanoma or a nevus by comparing the score to a classification threshold. Melanoma imaging biomarkers have been shown to be spectrally dependent in Red, Green, Blue (RGB) color channels, and hyperspectral imaging may further enhance diagnostic power. The purpose of this study was to use the same melanoma imaging biomarkers previously described, but over a wider range of wavelengths to determine if, in combination with machine learning algorithms, this could result in enhanced melanoma detection.
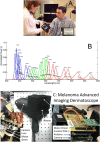
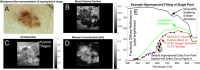
Methods: We used the melanoma advanced imaging dermatoscope (mAID) to image pigmented lesions assessed by dermatologists as requiring a biopsy. The mAID is a 21-wavelength imaging device in the 350-950 nm range. We then generated imaging biomarkers from these hyperspectral dermoscopy images, and, with the help of artificial intelligence algorithms, generated a melanoma Q-score for each lesion (0 = nevus, 1 = melanoma). The Q-score was then compared to the histopathologic diagnosis.
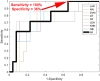
Results: The overall sensitivity and specificity of hyperspectral dermoscopy in detecting melanoma when evaluated in a set of lesions selected by dermatologists as requiring biopsy was 100% and 36%, respectively.
Conclusion: With widespread application, and if validated in larger clinical trials, this non-invasive methodology could decrease unnecessary biopsies and potentially increase life-saving early detection events. Lasers Surg. Med. 51:214-222, 2019. © 2019 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: artificial intelligence; dermoscopy; hyperspectral imaging; machine learning; melanoma.
© 2019 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Figures
References
-
- Salerni G, Terán T, Puig S, et al. Meta‐analysis of digital dermoscopy follow‐up of melanocytic skin lesions: A study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol 2013;27:805–814. - PubMed
-
- Friedman RJ, Gutkowicz‐Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer‐vision system on small‐diameter melanomas. Arch Dermatol 2008;144:476–482. - PubMed
-
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: Diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol 2018;29:1836–1842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

