HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells
- PMID: 30653763
- PMCID: PMC6590488
- DOI: 10.1002/ptr.6273
HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells
Abstract
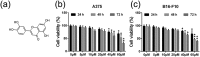
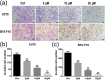
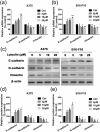
Tumor metastasis is still the leading cause of melanoma mortality. Luteolin, a natural flavonoid, is found in fruits, vegetables, and medicinal herbs. The pharmacological action and mechanism of luteolin on the metastasis of melanoma remain elusive. In this study, we investigated the effect of luteolin on A375 and B16-F10 cell viability, migration, invasion, adhesion, and tube formation of human umbilical vein endothelial cells. Epithelial-mesenchymal transition (EMT) markers and pivotal molecules in HIF-1α/VEGF signaling expression were analysed using western blot assays or quantitative real-time polymerase chain reaction. Results showed that luteolin inhibits cellular proliferation in A375 and B16-F10 melanoma cells in a time-dependent and concentration-dependent manner. Luteolin significantly inhibited the migratory, invasive, adhesive, and tube-forming potential of highly metastatic A375 and B16-F10 melanoma cells or human umbilical vein endothelial cells at sub-IC50 concentrations, where no significant cytotoxicity was observed. Luteolin effectively suppressed EMT by increased E-cadherin and decreased N-cadherin and vimentin expression both in mRNA and protein levels. Further, luteolin exerted its anti-metastasis activity through decreasing the p-Akt, HIF-1α, VEGF-A, p-VEGFR-2, MMP-2, and MMP-9 proteins expression. Overall, our findings first time suggests that HIF-1α/VEGF signaling-mediated EMT and angiogenesis is critically involved in anti-metastasis effect of luteolin as a potential therapeutic candidate for melanoma.
Keywords: HIF-1α/VEGF signaling; angiogenesis; epithelial-mesenchymal transition; luteolin; melanoma; metastasis.
© 2019 The Authors Phytotherapy Research Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

