Endometrial Epithelial Cell Apoptosis Is Inhibited by a ciR8073-miR181a-Neurotensis Pathway during Embryo Implantation
- PMID: 30654188
- PMCID: PMC6348770
- DOI: 10.1016/j.omtn.2018.12.005
Endometrial Epithelial Cell Apoptosis Is Inhibited by a ciR8073-miR181a-Neurotensis Pathway during Embryo Implantation
Abstract
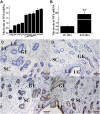
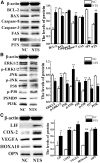
Development of the receptive endometrium (RE) from the pre-receptive endometrium (PE) is essential for embryo implantation, but its molecular mechanisms have not been fully understood. In this study, lncRNA-miRNA-mRNA and circRNA-miRNA-mRNA networks were constructed to explore the functions of potential competing endogenous RNAs (ceRNA) during the development of RE in dairy goats. We observed that circRNA8073 (ciR8073) decreased the levels of miR-181a by acting as a miRNA sponge. This effect indirectly increased the expression of neurotensin in endometrial epithelial cells (EECs). Neurotensin then inhibited EEC apoptosis by increasing the expression of BCL-2/BAX in favor of BCL-2 via the MAPK pathway and also induced increased expression of leukemia-inhibitory factor, cyclo-oxygenase 2, vascular endothelial growth factor A, and homeobox A10. We have thus identified a ciR8073-miR181a-neurotensin pathway in the endometrium of dairy goats. Through this pathway, ciR8073 functions as a ceRNA that sequesters miR-181a, thereby protecting neurotensin transcripts from miR-181a-mediated suppression in EECs.
Keywords: EECs; ceRNA; ciR8073-miR181a-NTS; dairy goats; endometrial epithelial cells; receptive endometrium.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures









Similar articles
-
Testin was regulated by circRNA3175-miR182 and inhibited endometrial epithelial cell apoptosis in pre-receptive endometrium of dairy goats.J Cell Physiol. 2018 Oct;233(10):6965-6974. doi: 10.1002/jcp.26614. Epub 2018 Apr 25. J Cell Physiol. 2018. PMID: 29693265
-
CircRNA-9119 regulates the expression of prostaglandin-endoperoxide synthase 2 (PTGS2) by sponging miR-26a in the endometrial epithelial cells of dairy goat.Reprod Fertil Dev. 2018 Nov;30(12):1759-1769. doi: 10.1071/RD18074. Reprod Fertil Dev. 2018. PMID: 29983139
-
MiR-184 Combined with STC2 Promotes Endometrial Epithelial Cell Apoptosis in Dairy Goats via RAS/RAF/MEK/ERK Pathway.Genes (Basel). 2020 Sep 7;11(9):1052. doi: 10.3390/genes11091052. Genes (Basel). 2020. PMID: 32906580 Free PMC article.
-
The role of apoptosis in human embryo implantation.J Reprod Immunol. 2015 Apr;108:114-22. doi: 10.1016/j.jri.2015.02.002. Epub 2015 Feb 28. J Reprod Immunol. 2015. PMID: 25779030 Review.
-
How to Create an Embryo Penetration Route.Am J Reprod Immunol. 2016 Mar;75(3):326-32. doi: 10.1111/aji.12476. Epub 2016 Jan 5. Am J Reprod Immunol. 2016. PMID: 26732539 Review.
Cited by
-
MicroRNAs, endometrial receptivity and molecular pathways.Reprod Biol Endocrinol. 2024 Nov 11;22(1):139. doi: 10.1186/s12958-024-01304-9. Reprod Biol Endocrinol. 2024. PMID: 39529197 Free PMC article. Review.
-
Construction of Circulating MicroRNAs-Based Non-invasive Prediction Models of Recurrent Implantation Failure by Network Analysis.Front Genet. 2021 Jul 23;12:712150. doi: 10.3389/fgene.2021.712150. eCollection 2021. Front Genet. 2021. PMID: 34367263 Free PMC article.
-
Neurotensin and Its Involvement in Female Hormone-Sensitive Cancers.Int J Mol Sci. 2024 Oct 30;25(21):11648. doi: 10.3390/ijms252111648. Int J Mol Sci. 2024. PMID: 39519199 Free PMC article. Review.
-
Neurotensin modulates the female reproductive system via extracellular signal-regulated kinase 1/2 signaling pathway: an in vivo and in silico study in mice.Mol Biol Rep. 2025 Jun 5;52(1):552. doi: 10.1007/s11033-025-10661-6. Mol Biol Rep. 2025. PMID: 40471330
-
Functional Role of circRNAs in the Regulation of Fetal Development, Muscle Development, and Lactation in Livestock.Biomed Res Int. 2021 Feb 19;2021:5383210. doi: 10.1155/2021/5383210. eCollection 2021. Biomed Res Int. 2021. PMID: 33688493 Free PMC article. Review.
References
-
- Revel A., Achache H., Stevens J., Smith Y., Reich R. MicroRNAs are associated with human embryo implantation defects. Hum. Reprod. 2011;26:2830–2840. - PubMed
-
- Macklon N.S., Stouffer R.L., Giudice L.C., Fauser B.C. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr. Rev. 2006;27:170–207. - PubMed
-
- Shahandeh A. Molecular mechanisms of oncogenic long non-coding RNAs. Bioscience Research. 2013;10:38–54.
-
- Thum T., Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ. Res. 2015;116:751–762. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

