Implantable antimicrobial biomaterials for local drug delivery in bone infection models
- PMID: 30654212
- PMCID: PMC6615972
- DOI: 10.1016/j.actbio.2019.01.015
Implantable antimicrobial biomaterials for local drug delivery in bone infection models
Abstract
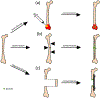
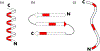
Increased use of implantable biomedical devices demonstrates their potential in treating a wide variety of ailments and disorders in bone trauma and orthopaedic, reconstructive, and craniofacial applications. However, the number of cases involving implant failure or malfunction due to bacterial infection have also increased in recent years. Implanted devices can facilitate the growth of bacteria as these micro-organisms have the potential to adhere to the implant and grow and develop to form biofilms. In an effort to better understand and mitigate these occurrences, biomaterials containing antimicrobial agents that can be released or presented within the local microenvironment have become an important area of research. In this review, we discuss critical factors that regulate antimicrobial therapy to sites of bone infection, such as key biomolecular considerations and platforms for delivery, as well as current in vivo models and current advances in the field. STATEMENT OF SIGNIFICANCE: This review outlines the important factors that are taken into consideration for the development of biomaterials for local delivery of therapeutics to the site of bone infections. An overview of important criteria for development of this model (such as type of bone defect, antimicrobial therapeutic, and delivery vehicle) are provided, along with current research that utilizes these considerations. Additionally, this review highlights recent clinical trials that have utilized antimicrobial therapeutics for treatment of osteomyelitis.
Keywords: Antimicrobial; Bone defect; Implantable biomaterials; Local delivery; Orthopaedic infection.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures
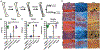
Similar articles
-
Prevention of microbial biofilms - the contribution of micro and nanostructured materials.Curr Med Chem. 2014;21(29):3311. doi: 10.2174/0929867321666140304101314. Curr Med Chem. 2014. PMID: 24606506
-
Selecting anti-infective agents for the treatment of bone infections.Orthopedics. 2007 Sep;30(9):713-7. doi: 10.3928/01477447-20070901-26. Orthopedics. 2007. PMID: 17899911 Review. No abstract available.
-
Recent Advances and Future Prospects on Adaptive Biomaterials for Antimicrobial Applications.Macromol Biosci. 2019 Dec;19(12):e1900289. doi: 10.1002/mabi.201900289. Epub 2019 Oct 23. Macromol Biosci. 2019. PMID: 31642591 Review.
-
Advances in immunotherapy delivery from implantable and injectable biomaterials.Acta Biomater. 2019 Apr 1;88:15-31. doi: 10.1016/j.actbio.2019.02.016. Epub 2019 Feb 13. Acta Biomater. 2019. PMID: 30771535 Free PMC article. Review.
-
Orthopaedic biofilm infections.APMIS. 2017 Apr;125(4):353-364. doi: 10.1111/apm.12687. APMIS. 2017. PMID: 28407423 Review.
Cited by
-
Application of loaded graphene oxide biomaterials in the repair and treatment of bone defects.Bone Joint Res. 2024 Dec 5;13(12):725-740. doi: 10.1302/2046-3758.1312.BJR-2024-0048.R1. Bone Joint Res. 2024. PMID: 39631429 Free PMC article.
-
Biomaterial-Based Scaffolds as Carriers of Topical Antimicrobials for Bone Infection Prophylaxis.Pol J Microbiol. 2025 Jun 18;74(2):232-243. doi: 10.33073/pjm-2025-019. eCollection 2025 Jun 1. Pol J Microbiol. 2025. PMID: 40544521 Free PMC article. Review.
-
3D Printing and Electrospinning of Drug- and Graphene-Enhanced Polycaprolactone Scaffolds for Osteochondral Nasal Repair.Materials (Basel). 2025 Apr 16;18(8):1826. doi: 10.3390/ma18081826. Materials (Basel). 2025. PMID: 40333487 Free PMC article.
-
Magnetite Nanoparticles and Essential Oils Systems for Advanced Antibacterial Therapies.Int J Mol Sci. 2020 Oct 5;21(19):7355. doi: 10.3390/ijms21197355. Int J Mol Sci. 2020. PMID: 33027980 Free PMC article. Review.
-
Recent advances in surface-mounted metal-organic framework thin film coatings for biomaterials and medical applications: a review.Biomater Res. 2023 Nov 10;27(1):115. doi: 10.1186/s40824-023-00454-y. Biomater Res. 2023. PMID: 37950330 Free PMC article. Review.
References
-
- Lind KD, Understanding the market for implantable medical devices, AARP Insight on the Issues 129 (2018).
-
- Moriarty TF, Zaat SAJ, & Busscher HJ, Biomaterials associated infection: Immunological aspects and antimicrobial strategies, Springer; (2013).
-
- Gomes D, Pereira M, Bettencourt AF, Osteomyelitis: an overview of antimicrobial therapy, Brazilian Journal of Pharmaceutical Sciences 49 (2013) 13–27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

