3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice
- PMID: 30654329
- PMCID: PMC6366991
- DOI: 10.18632/aging.101748
3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice
Abstract
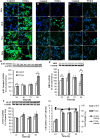
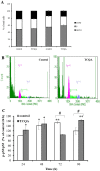
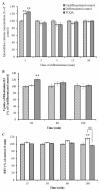
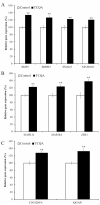
Caffeoylquinic acid (CQA) is a natural polyphenol with evidence of antioxidant and neuroprotective effects and prevention of deficits in spatial learning and memory. We studied the cognitive-enhancing effect of 3,4,5-tricaffeoylquinic acid (TCQA) and explored its cellular and molecular mechanism in the senescence-accelerated mouse prone 8 (SAMP8) model of aging and Alzheimer's disease as well as in human neural stem cells (hNSCs). Mice were fed with 5 mg/kg of TCQA for 30 days and were tested in the Morris water maze (MWM). Brain tissues were collected for immunohistochemical detection of bromodeoxyuridine (BrdU) to detect activated stem cells and newborn neurons. TCQA-treated SAMP8 exhibited significantly improved cognitive performance in MWM compared to water-treated SAMP8. TCQA-treated SAMP8 mice also had significantly higher numbers of BrdU+/glial fibrillary acidic protein (GFAP+) and BrdU+/Neuronal nuclei (NeuN+) cells in the dentate gyrus (DG) neurogenic niche compared with untreated SAMP8. In hNSCs, TCQA induced cell cycle arrest at G0/G1, actin cytoskeleton organization, chromatin remodeling, neuronal differentiation, and bone morphogenetic protein signaling. The neurogenesis promoting effect of TCQA in the DG of SAMP8 mice might explain the cognition-enhancing influence of TCQA observed in our study, and our hNSCs in aggregate suggest a therapeutic potential for TCQA in aging-associated diseases.
Keywords: BMP signaling; SAMP8; TCQA; neurogenesis; spatial learning and memory.
Conflict of interest statement
Figures
Similar articles
-
Microalgae Aurantiochytrium Sp. Increases Neurogenesis and Improves Spatial Learning and Memory in Senescence-Accelerated Mouse-Prone 8 Mice.Front Cell Dev Biol. 2021 Feb 9;8:600575. doi: 10.3389/fcell.2020.600575. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33634096 Free PMC article.
-
Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1.Neuroscience. 2010 Sep 1;169(3):1039-45. doi: 10.1016/j.neuroscience.2010.05.049. Epub 2010 Jun 4. Neuroscience. 2010. PMID: 20570715
-
Limited hippocampal neurogenesis in SAMP8 mouse model of Alzheimer's disease.Brain Res. 2011 May 10;1389:183-93. doi: 10.1016/j.brainres.2011.03.039. Epub 2011 Mar 22. Brain Res. 2011. PMID: 21439270
-
The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer's disease.Exp Gerontol. 2005 Oct;40(10):774-83. doi: 10.1016/j.exger.2005.05.007. Epub 2005 Jul 18. Exp Gerontol. 2005. PMID: 16026957 Review.
-
Age-related cognitive decline: can neural stem cells help us?Aging (Albany NY). 2012 Mar;4(3):176-86. doi: 10.18632/aging.100446. Aging (Albany NY). 2012. PMID: 22466406 Free PMC article. Review.
Cited by
-
Regulating cell fate of human amnion epithelial cells using natural compounds: an example of enhanced neural and pigment differentiation by 3,4,5-tri-O-caffeoylquinic acid.Cell Commun Signal. 2021 Feb 24;19(1):26. doi: 10.1186/s12964-020-00697-5. Cell Commun Signal. 2021. PMID: 33627134 Free PMC article.
-
Chrysanthemum coronarium L. Protects against Premature Senescence in Human Endothelial Cells.Curr Issues Mol Biol. 2022 Nov 23;44(12):5839-5847. doi: 10.3390/cimb44120397. Curr Issues Mol Biol. 2022. PMID: 36547058 Free PMC article.
-
Antidepressant-Like Effect of Ferulic Acid via Promotion of Energy Metabolism Activity.Mol Nutr Food Res. 2019 Oct;63(19):e1900327. doi: 10.1002/mnfr.201900327. Epub 2019 Aug 22. Mol Nutr Food Res. 2019. PMID: 31394019 Free PMC article.
-
3,4,5-Tri-O-Caffeoylquinic Acid Promoted Hair Pigmentation Through β-Catenin and Its Target Genes.Front Cell Dev Biol. 2020 Mar 25;8:175. doi: 10.3389/fcell.2020.00175. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32269993 Free PMC article.
-
Microalgae Aurantiochytrium Sp. Increases Neurogenesis and Improves Spatial Learning and Memory in Senescence-Accelerated Mouse-Prone 8 Mice.Front Cell Dev Biol. 2021 Feb 9;8:600575. doi: 10.3389/fcell.2020.600575. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33634096 Free PMC article.
References
-
- World Population Ageing United Nations Department of Economic and Social Affairs, Population Division. 2015 ST/ESA/SER.A/390.
-
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

