Effects of N-Acetyl-Cysteine Supplementation through Drinking Water on the Glutathione Redox Status during the Weaning Transition of Piglets
- PMID: 30654433
- PMCID: PMC6356391
- DOI: 10.3390/antiox8010024
Effects of N-Acetyl-Cysteine Supplementation through Drinking Water on the Glutathione Redox Status during the Weaning Transition of Piglets
Abstract
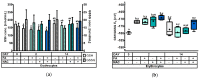
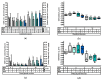
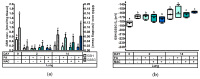
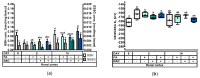
This study investigated the effect of N-acetyl-cysteine (NAC) supplementation through drinking water on animal performance and the glutathione (GSH) redox system in weaned piglets, particularly in relation to the immediate post-weaning feed intake. To this end, 168 piglets were weaned and either fed ad libitum or fasted the first two days, and either or not administered 200 mg/L NAC via the drinking water until d14 post-weaning. Next to animal performance until day 42 (d42), the GSH redox system was measured in erythrocytes, small intestinal mucosa, liver, lung, and kidney tissue at d0, d2, and d14 post-weaning. Animal performance and GSH levels were not affected by NAC, nor by fasting. Irrespective of treatment, a significant drop in GSH at d2 post-weaning was found as compared to d0, in particular in liver (-69%), distal jejunal mucosa (-72%), and lung tissue (-80%). Post-weaning changes of the GSH redox status were strongly tissue-dependent. To conclude, this research indicates that GSH redox homeostasis was largely affected in multiple organs during the weaning transition. NAC supplementation did not increase GSH levels in any tissue, not even in fasted animals, questioning the fact if cysteine is the first or only limiting factor determining the rate of GSH synthesis in the early post-weaning phase.
Keywords: N-acetyl-cysteine; glutathione; redox status; small intestine; weaned pigs.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

