Cellular and Molecular Mechanisms Mediated by recPrPC Involved in the Neuronal Differentiation Process of Mesenchymal Stem Cells
- PMID: 30654447
- PMCID: PMC6358746
- DOI: 10.3390/ijms20020345
Cellular and Molecular Mechanisms Mediated by recPrPC Involved in the Neuronal Differentiation Process of Mesenchymal Stem Cells
Abstract
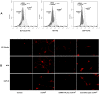
Human Dental Pulp Stem Cells (hDPSCs) represent a type of adult mesenchymal stem cells that have the ability to differentiate in vitro in several lineages such as odontoblasts, osteoblasts, chondrocytes, adipocytes and neurons. In the current work, we used hDPSCs as the experimental model to study the role of recombinant prion protein 23⁻231 (recPrPC) in the neuronal differentiation process, and in the signal pathway activation of ERK 1/2 and Akt. We demonstrated that recPrPC was able to activate an intracellular signal pathway mediated by extracellular-signal-regulated kinase 1 and 2 (ERK 1/2) and protein kinase B (Akt). Moreover, in order to understand whether endogenous prion protein (PrPC) was necessary to mediate the signaling induced by recPrPC, we silenced PrPC, demonstrating that the presence of endogenous PrPC was essential for ERK 1/2 and Akt phosphorylation. Since endogenous PrPC is a well-known lipid rafts component, we evaluated the role of these structures in the signal pathway induced by recPrPC. Our results suggest that lipid rafts integrity play a key role in recPrPC activity. In fact, lipid rafts inhibitors, such as fumonisin B1 and MβCD, significantly prevented ERK 1/2 and Akt phosphorylation induced by recPrPC. In addition, we investigated the capacity of recPrPC to induce hDPSCs neuronal differentiation process after long-term stimulation through the evaluation of typical neuronal markers expression such as B3-Tubulin, neurofilament-H (NFH) and growth associated protein 43 (GAP43). Accordingly, when we silenced endogenous PrPC, we observed the inhibition of neuronal differentiation induced by recPrPC. The combined data suggest that recPrPC plays a key role in the neuronal differentiation process and in the activation of specific intracellular signal pathways in hDPSCs.
Keywords: adult neurogenesis; cellular prion protein; dental pulp-derived stem cells; lipid rafts; mesenchymal stem cells; neural stem cells; neuronal differentiation; prions; recombinant prion protein; shed prion protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

