Hibernation and Radioprotection: Gene Expression in the Liver and Testicle of Rats Irradiated under Synthetic Torpor
- PMID: 30654467
- PMCID: PMC6359347
- DOI: 10.3390/ijms20020352
Hibernation and Radioprotection: Gene Expression in the Liver and Testicle of Rats Irradiated under Synthetic Torpor
Abstract
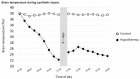
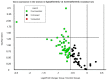
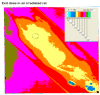
Hibernation has been proposed as a tool for human space travel. In recent years, a procedure to induce a metabolic state known as "synthetic torpor" in non-hibernating mammals was successfully developed. Synthetic torpor may not only be an efficient method to spare resources and reduce psychological problems in long-term exploratory-class missions, but may also represent a countermeasure against cosmic rays. Here we show the preliminary results from an experiment in rats exposed to ionizing radiation in normothermic conditions or synthetic torpor. Animals were irradiated with 3 Gy X-rays and organs were collected 4 h after exposure. Histological analysis of liver and testicle showed a reduced toxicity in animals irradiated in torpor compared to controls irradiated at normal temperature and metabolic activity. The expression of ataxia telangiectasia mutated (ATM) in the liver was significantly downregulated in the group of animal in synthetic torpor. In the testicle, more genes involved in the DNA damage signaling were downregulated during synthetic torpor. These data show for the first time that synthetic torpor is a radioprotector in non-hibernators, similarly to natural torpor in hibernating animals. Synthetic torpor can be an effective strategy to protect humans during long term space exploration of the solar system.
Keywords: ATM; hibernation; hypothermia; liver; radiation; raphe pallidus; space exploration; synthetic torpor; testicle; torpor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

