Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings
- PMID: 30654468
- PMCID: PMC6358940
- DOI: 10.3390/ijms20020353
Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings
Abstract
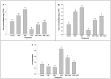
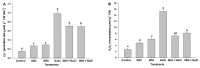
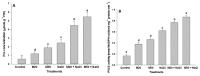
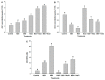
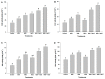
Melatonin, a natural agent, has multiple functions in animals as well as in plants. However, its possible roles in plants under abiotic stress are not clear. Nowadays, soil salinity is a major threat to global agriculture because a high soil salt content causes multiple stresses (hyperosmotic, ionic, and oxidative). Therefore, the aim of the present study was to explore: (1) the involvement of melatonin in biosynthesis of photosynthetic pigments and in regulation of photosynthetic enzymes, such as carbonic anhydrase (CA) and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco); (2) the role of melatonin in osmoregulation by proline and carbohydrate metabolism; and (3) the function of melatonin in the antioxidant defense system under salinity. Outcomes of the study reveal that under non-saline conditions, application of melatonin (20 and 50 µM) improved plant growth, viz. shoot length, root length, shoot fresh weight (FW), root FW, shoot dry weight (DW), root DW and leaf area and physio-biochemical parameters [chlorophyll (Chl) a and b, proline (Pro) and total soluble carbohydrates (TSC) content, and increased the activity of CA and Rubisco]. However, tomato seedlings treated with NaCl exhibited enhanced Chl degradation, electrolyte leakage (EL), malondialdehyde (MDA) and reactive oxygen species (ROS; superoxide and hydrogen peroxide). ROS were detected in leaf and root. Interestingly, application of melatonin improved plant growth and reduced EL, MDA and ROS levels through upregulation of photosynthesis enzymes (CA, Rubisco), antioxidant enzymes (superoxide dismutase, catalase, glutathione reductase and ascorbate reductase) and levels of non-enzymatic antioxidants [ascorbate (ASC) and reduced glutathione (GSH)], as well as by affecting the ASC-GSH cycle. Additionally, exogenous melatonin also improved osmoregulation by increasing the content of TSC, Pro and Δ¹-pyrroline-5-carboxylate synthetase activity. These results suggest that melatonin has beneficial effects on tomato seedlings growth under both stress and non-stress conditions. Melatonin's role in tolerance to salt stress may be associated with the regulation of enzymes involved in photosynthesis, the antioxidant system, metabolism of proline and carbohydrate, and the ASC-GSH cycle. Also, melatonin could be responsible for maintaining the high ratios of GSH/GSSG and ASC/DHA.
Keywords: ASC-GSH pathway; SOD-CAT pathway; Solanum lycopersicum; antioxidant system; carbohydrate; melatonin; proline; Δ1-pyrroline-5-carboxylate synthetase.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


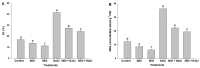


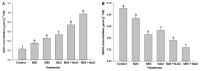
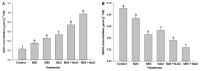

References
-
- Siddiqui M.H., Alamri S.A., Al-Khaishany M.Y., Al-Qutami M.A., Ali H.M., AL-Rabiah H., Kalaji H.M. Exogenous application of nitric oxide and spermidine reduces the negative effects of salt stress on tomato. Hortic. Environ. Biotechnol. 2017;58:537–547. doi: 10.1007/s13580-017-0353-4. - DOI
-
- Carillo P., Cirillo C., De Micco V., Arena C., De Pascale S., Rouphael Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2019;212:12–22. doi: 10.1016/j.agwat.2018.08.037. - DOI
-
- Siddiqui M.H., Mohammad F., Khan M.N. Morphological and physio-biochemical characterization of Brassica juncea L. Czern. & Coss. genotypes under salt stress. J. Plant Interact. 2009;4:67–80.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

