Validity of an Abbreviated, Clinically Feasible Test for Postprandial Lipemia in Healthy Adults: A Randomized Cross-Over Study
- PMID: 30654471
- PMCID: PMC6356282
- DOI: 10.3390/nu11010180
Validity of an Abbreviated, Clinically Feasible Test for Postprandial Lipemia in Healthy Adults: A Randomized Cross-Over Study
Abstract
Background: A large post-meal triglyceride (TG) response is an independent risk factor for cardiovascular disease, but postprandial lipemia assessments are not clinically practical in their current form. Therefore, we assessed the validity of an abbreviated, clinically feasible protocol in measuring postprandial lipemia.
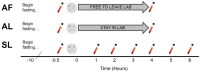
Method: Eighteen healthy adults (8 male and 10 female) completed 3 high-fat meal trials in random order: (1) a Standard in Lab (SL) protocol wherein blood draws (to determine TG) were made from a catheter at baseline and hourly for 6 h; (2) an Abbreviated in Lab (AL) protocol in which participants remained in the laboratory but blood draws were only made at baseline and 4 h post-meal; and (3) an Abbreviated with Freedom (AF) protocol in which participants vacated the laboratory between the meal and the 4-h blood draw.
Results: TG increase from baseline was very similar (p = 0.93) across the 3 trials (SL: 68.5 ± 62.7 mg/dL; AL: 71.1 ± 58.0 mg/dL; AF: 66.7 ± 46.4 mg/dL), as were 4-h TG levels (SL: 144.6 ± 84.2 mg/dL; AL: 171.4 ± 88.2 mg/dL; AF: 157.7 ± 76.7 mg/dL; p = 0.49). Similarly, total and incremental area under the curve (AUC) were not significantly different across the trials (p = 0.12 and 0.91, respectively).
Conclusion: The TG results of the clinically feasible, abbreviated protocol were similar to those of the more exhaustive standard protocol. The AF protocol could be a valid and feasible clinical tool for measurement of postprandial lipemia and assessment of cardiovascular risk, although studies in larger and more diverse cohorts are needed.
Keywords: fat tolerance; post-meal; risk assessment; triglycerides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Guerci B., Paul J., Hadjadj S., Durlach V., Vergčs B., Attia N., Girard-Globa A., Drouin P. Analysis of the postprandial lipid metabolism: Use of a 3-point test. Diabetes Metab. 2008;27:449–457. - PubMed
-
- Rector S.R., Linden M.A., Zhang J.Q., Warner S.O., Altena T.S., Smith B.K., Ziogas G.G., Liu Y., Thomas T.R. Predicting Postprandial Lipemia in Healthy Adults and in At-Risk Individuals with Components of the Cardiometabolic Syndrome. J. Clin. Hypertens. 2009;11:663–671. doi: 10.1111/j.1559-4572.2008.00026.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

