Textile-Based Potentiometric Electrochemical pH Sensor for Wearable Applications
- PMID: 30654478
- PMCID: PMC6468877
- DOI: 10.3390/bios9010014
Textile-Based Potentiometric Electrochemical pH Sensor for Wearable Applications
Abstract
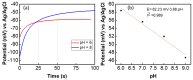
In this work, we present a potentiometric pH sensor on textile substrate for wearable applications. The sensitive (thick film graphite composite) and reference electrodes (Ag/AgCl) are printed on cellulose-polyester blend cloth. An excellent adhesion between printed electrodes allow the textile-based sensor to be washed with a reliable pH response. The developed textile-based pH sensor works on the basis of electrochemical reaction, as observed through the potentiometric, cyclic voltammetry (100 mV/s) and electrochemical impedance spectroscopic (10 mHz to 1 MHz) analysis. The electrochemical double layer formation and the ionic exchanges of the sensitive electrode-pH solution interaction are observed through the electrochemical impedance spectroscopic analysis. Potentiometric analysis reveals that the fabricated textile-based sensor exhibits a sensitivity (slope factor) of 4 mV/pH with a response time of 5 s in the pH range 6⁻9. The presented sensor shows stable response with a potential of 47 ± 2 mV for long time (2000 s) even after it was washed in tap water. These results indicate that the sensor can be used for wearable applications.
Keywords: e-textile; graphite; pH sensor; potentiometric; wearable system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Emaminejad S., Gao W., Wu E., Davies Z.A., Yin Yin Nyein H., Challa S., Ryan S.P., Fahad H.M., Chen K., Shahpar Z., et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA. 2017;114:4625–4630. doi: 10.1073/pnas.1701740114. - DOI - PMC - PubMed
-
- Manjakkal L., Sakthivel B., Gopalakrishnan N., Dahiya R. Printed flexible electrochemical pH sensors based on CuO nanorods. Sens. Actuator B Chem. 2018;263:50–58. doi: 10.1016/j.snb.2018.02.092. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

