Regulated Assembly of LPS, Its Structural Alterations and Cellular Response to LPS Defects
- PMID: 30654491
- PMCID: PMC6358824
- DOI: 10.3390/ijms20020356
Regulated Assembly of LPS, Its Structural Alterations and Cellular Response to LPS Defects
Abstract
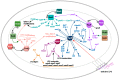
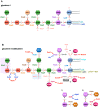
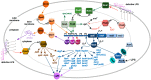
Distinguishing feature of the outer membrane (OM) of Gram-negative bacteria is its asymmetry due to the presence of lipopolysaccharide (LPS) in the outer leaflet of the OM and phospholipids in the inner leaflet. Recent studies have revealed the existence of regulatory controls that ensure a balanced biosynthesis of LPS and phospholipids, both of which are essential for bacterial viability. LPS provides the essential permeability barrier function and act as a major virulence determinant. In Escherichia coli, more than 100 genes are required for LPS synthesis, its assembly at inner leaflet of the inner membrane (IM), extraction from the IM, translocation to the OM, and in its structural alterations in response to various environmental and stress signals. Although LPS are highly heterogeneous, they share common structural elements defining their most conserved hydrophobic lipid A part to which a core polysaccharide is attached, which is further extended in smooth bacteria by O-antigen. Defects or any imbalance in LPS biosynthesis cause major cellular defects, which elicit envelope responsive signal transduction controlled by RpoE sigma factor and two-component systems (TCS). RpoE regulon members and specific TCSs, including their non-coding arm, regulate incorporation of non-stoichiometric modifications of LPS, contributing to LPS heterogeneity and impacting antibiotic resistance.
Keywords: LapB; Lpt transport system; LpxC; Rcs two-component system; RpoE sigma factor; lipid A modifications; lipid IVA; noncoding small regulatory RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nikaido H. Outer membrane. In: Neidhardt F.C., Curtiss R., Ingraham J.L., editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd ed. Vol. 1. American Society for Microbiology Press; Washington, DC, USA: 1996. pp. 29–47.
-
- Anderson M.S., Bulawa C.E., Raetz C.R.H. The biosynthesis of Gram-negative endotoxin. Formation of lipid A precursors from UDP-GlcNAc in extracts of Escherichia coli. J. Biol. Chem. 1985;260:15536–15541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

