Aquaporins in Renal Diseases
- PMID: 30654539
- PMCID: PMC6359174
- DOI: 10.3390/ijms20020366
Aquaporins in Renal Diseases
Abstract
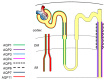
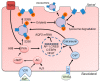
Aquaporins (AQPs) are a family of highly selective transmembrane channels that mainly transport water across the cell and some facilitate low-molecular-weight solutes. Eight AQPs, including AQP1, AQP2, AQP3, AQP4, AQP5, AQP6, AQP7, and AQP11, are expressed in different segments and various cells in the kidney to maintain normal urine concentration function. AQP2 is critical in regulating urine concentrating ability. The expression and function of AQP2 are regulated by a series of transcriptional factors and post-transcriptional phosphorylation, ubiquitination, and glycosylation. Mutation or functional deficiency of AQP2 leads to severe nephrogenic diabetes insipidus. Studies with animal models show AQPs are related to acute kidney injury and various chronic kidney diseases, such as diabetic nephropathy, polycystic kidney disease, and renal cell carcinoma. Experimental data suggest ideal prospects for AQPs as biomarkers and therapeutic targets in clinic. This review article mainly focuses on recent advances in studying AQPs in renal diseases.
Keywords: acute kidney injury; aquaporin; diabetic nephropathy; nephrogenic diabetes insipidus; polycystic kidney disease; renal cell carcinoma; vasopressin.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Aquaporins in the kidney: physiology and pathophysiology.Am J Physiol Renal Physiol. 2020 Jan 1;318(1):F193-F203. doi: 10.1152/ajprenal.00304.2019. Epub 2019 Nov 4. Am J Physiol Renal Physiol. 2020. PMID: 31682170 Review.
-
Renal aquaporins and water balance disorders.Biochim Biophys Acta. 2014 May;1840(5):1533-49. doi: 10.1016/j.bbagen.2013.12.002. Epub 2013 Dec 15. Biochim Biophys Acta. 2014. PMID: 24342488 Review.
-
The role of renal water channels in health and disease.Mol Aspects Med. 2012 Oct-Dec;33(5-6):547-52. doi: 10.1016/j.mam.2012.01.001. Epub 2012 Jan 12. Mol Aspects Med. 2012. PMID: 22252122 Free PMC article. Review.
-
Aquaporins in Urinary System.Adv Exp Med Biol. 2023;1398:155-177. doi: 10.1007/978-981-19-7415-1_11. Adv Exp Med Biol. 2023. PMID: 36717493
-
Roles of aquaporins in kidney revealed by transgenic mice.Semin Nephrol. 2006 May;26(3):200-8. doi: 10.1016/j.semnephrol.2006.02.002. Semin Nephrol. 2006. PMID: 16713493 Review.
Cited by
-
Abnormal expression and the significant prognostic value of aquaporins in clear cell renal cell carcinoma.PLoS One. 2022 Mar 4;17(3):e0264553. doi: 10.1371/journal.pone.0264553. eCollection 2022. PLoS One. 2022. PMID: 35245343 Free PMC article.
-
Predictive Value of Urinary Aquaporin 2 for Acute Kidney Injury in Patients with Acute Decompensated Heart Failure.Biomedicines. 2022 Mar 6;10(3):613. doi: 10.3390/biomedicines10030613. Biomedicines. 2022. PMID: 35327416 Free PMC article.
-
The Effect of Shen Qi Wan Medicated Serum on NRK-52E Cells Proliferation and Migration by Targeting Aquaporin 1 (AQP1).Med Sci Monit. 2020 Jun 3;26:e922943. doi: 10.12659/MSM.922943. Med Sci Monit. 2020. PMID: 32491998 Free PMC article.
-
Roxadustat (FG-4592) Facilitates Recovery From Renal Damage by Ameliorating Mitochondrial Dysfunction Induced by Folic Acid.Front Pharmacol. 2022 Feb 25;12:788977. doi: 10.3389/fphar.2021.788977. eCollection 2021. Front Pharmacol. 2022. PMID: 35280255 Free PMC article.
-
Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice.Biomolecules. 2022 Dec 29;13(1):66. doi: 10.3390/biom13010066. Biomolecules. 2022. PMID: 36671451 Free PMC article.
References
-
- Geng X., Yang B. Transport Characteristics of Aquaporins. Adv. Exp. Med. Biol. 2017;969:51–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

