Olanzapine Versus Placebo in Adult Outpatients With Anorexia Nervosa: A Randomized Clinical Trial
- PMID: 30654643
- PMCID: PMC7015155
- DOI: 10.1176/appi.ajp.2018.18101125
Olanzapine Versus Placebo in Adult Outpatients With Anorexia Nervosa: A Randomized Clinical Trial
Erratum in
-
Correction to Attia et al.Am J Psychiatry. 2019 Jun 1;176(6):489. doi: 10.1176/appi.ajp.2019.1766correction1. Am J Psychiatry. 2019. PMID: 31154818 No abstract available.
Abstract
Objective: This study evaluated the benefits of olanzapine compared with placebo for adult outpatients with anorexia nervosa.
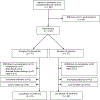
Methods: This randomized double-blind placebo-controlled trial of adult outpatients with anorexia nervosa (N=152, 96% of whom were women; the sample's mean body mass index [BMI] was 16.7) was conducted at five sites in North America. Participants were randomly assigned in a 1:1 ratio to receive olanzapine or placebo and were seen weekly for 16 weeks. The primary outcome measures were rate of change in body weight and rate of change in obsessionality, assessed with the Yale-Brown Obsessive Compulsive Scale (YBOCS).
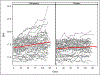
Results: Seventy-five participants were assigned to receive olanzapine and 77 to receive placebo. A statistically significant treatment-by-time interaction was observed, indicating that the increase in BMI over time was greater in the olanzapine group (0.259 [SD=0.051] compared with 0.095 [SD=0.053] per month). There was no significant difference between treatment groups in change in the YBOCS obsessions subscale score over time (-0.325 compared with -0.017 points per month) and there were no significant differences between groups in the frequency of abnormalities on blood tests assessing potential metabolic disturbances.
Conclusions: This study documented a modest therapeutic effect of olanzapine compared with placebo on weight in adult outpatients with anorexia nervosa, but no significant benefit for psychological symptoms. Nevertheless, the finding on weight is notable, as achieving change in weight is notoriously challenging in this disorder.
Trial registration: ClinicalTrials.gov NCT01170117.
Keywords: Antipsychotics; Clinical Drug Studies; Eating Disorders.
Figures
References
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition Arlington,VA, American Psychiatric Association; 2013.
-
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality Rates in Patients With Anorexia Nervosa and Other Eating Disorders: A Meta-analysis of 36 Studies. Archives of General Psychiatry. 2011;68:724–731. - PubMed
-
- Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, Pike KM, Devlin MJ, Woodside B, Roberto CA, Rockert W. Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA. 2006;295:2605–2612. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical