Development and Validation of the Real-World Progression in Diabetes (RAPIDS) Model
- PMID: 30654704
- PMCID: PMC6624067
- DOI: 10.1177/0272989X18817521
Development and Validation of the Real-World Progression in Diabetes (RAPIDS) Model
Abstract
Introduction: To develop and validate the first real-world data-based type 2 diabetes progression model (RAPIDS) employing econometric techniques that can study the comparative effects of complex dynamic patterns of glucose-lowering drug use.
Methods: The US Department of Veterans Affairs (VA) electronic medical record and claims databases were used to identify over 500,000 diabetes patients in 2003 with up to 9-year follow-up. The RAPIDS model contains interdependent first-order Markov processes over quarters for each of the micro- and macrovascular events, hypoglycemia, and death, as well as predictive models for 8 biomarker levels. Model parameters varied by static demographic factors and dynamic factors, such as age, duration of diabetes, 13 possible glucose-lowering treatment combinations, any blood pressure and any cholesterol-lowering medications, and cardiovascular history. To illustrate model capabilities, a simple comparative study was set up to compare observed treatment use patterns to alternate patterns if perfect adherence is assumed following initiating the use of any of these medications.
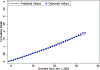
Results: Data were randomly split into 307,288, 105,195, and 105,081 patients to perform estimation, out-of-sample calibration, and validation, respectively. Model predictions in the validation sample closely aligned with the observed longitudinal trajectory of biomarkers and outcomes. Perfect adherence among initiators increased proportion of days covered by only 6 months. Most of this increase came from increased adherence to monotherapies and did not lead to meaningful changes in any of the outcomes over the 9-year period.
Conclusion: Future value of increasing medication adherence among VA patients with diabetes may lie among those who never initiate treatment or are late in initiating treatment. The first-of-its-kind real-world data-based model has the potential to carry out many complex comparative-effectiveness research (CER) studies of dynamic glucose-lowering drug regimens.
Keywords: comparative effectiveness research; diabetes; dynamic patterns; glucose-lowering; model; real-world data.
Figures




Similar articles
-
The Impact of Dual Health Care System Use for Obtaining Prescription Medications on Nonadherence in Veterans With Type 2 Diabetes.Ann Pharmacother. 2019 Jul;53(7):675-682. doi: 10.1177/1060028019828681. Epub 2019 Feb 6. Ann Pharmacother. 2019. PMID: 30724092
-
Glucose control and medication adherence among veterans with diabetes and serious mental illness: does collocation of primary care and mental health care matter?Diabetes Care. 2014 Aug;37(8):2261-7. doi: 10.2337/dc13-0051. Epub 2014 May 30. Diabetes Care. 2014. PMID: 24879839
-
Comparative Effectiveness of Insulin versus Combination Sulfonylurea and Insulin: a Cohort Study of Veterans with Type 2 Diabetes.J Gen Intern Med. 2016 Jun;31(6):638-46. doi: 10.1007/s11606-016-3633-2. J Gen Intern Med. 2016. PMID: 26921160 Free PMC article.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
-
Glucose-lowering medications and the risk of cancer: A methodological review of studies based on real-world data.Diabetes Obes Metab. 2019 Sep;21(9):2029-2038. doi: 10.1111/dom.13766. Epub 2019 May 29. Diabetes Obes Metab. 2019. PMID: 31062453 Free PMC article.
Cited by
-
Development and validation of a type 2 diabetes model to estimate the cost-effectiveness of diabetes interventions across the care continuum.Int J Technol Assess Health Care. 2025 Jun 2;41(1):e36. doi: 10.1017/S0266462325100172. Int J Technol Assess Health Care. 2025. PMID: 40452371 Free PMC article.
-
A Patient-Level Model to Estimate Lifetime Health Outcomes of Patients With Type 1 Diabetes.Diabetes Care. 2020 Aug;43(8):1741-1749. doi: 10.2337/dc19-2249. Epub 2020 Jun 12. Diabetes Care. 2020. PMID: 32532756 Free PMC article.
-
The sequence of disease-modifying anti-rheumatic drugs: pathways to and predictors of tocilizumab monotherapy.Arthritis Res Ther. 2021 Jan 14;23(1):26. doi: 10.1186/s13075-020-02408-4. Arthritis Res Ther. 2021. PMID: 33446261 Free PMC article.
-
Development and Validation of the US Diabetes, Obesity, Cardiovascular Disease Microsimulation (DOC-M) Model: Health Disparity and Economic Impact Model.Med Decis Making. 2023 Oct-Nov;43(7-8):930-948. doi: 10.1177/0272989X231196916. Epub 2023 Oct 16. Med Decis Making. 2023. PMID: 37842820 Free PMC article.
-
Updating and calibrating the Real-World Progression In Diabetes (RAPIDS) model in a non-Veterans Affairs population.Diabetes Obes Metab. 2024 Nov;26(11):5261-5271. doi: 10.1111/dom.15878. Epub 2024 Sep 2. Diabetes Obes Metab. 2024. PMID: 39223846 Free PMC article.
References
-
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999;281(21):2005–12. - PubMed
-
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298(2):194–206. - PubMed
-
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008;31(1):173–5. - PubMed
-
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358(24):2560–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

