VCP maintains lysosomal homeostasis and TFEB activity in differentiated skeletal muscle
- PMID: 30654731
- PMCID: PMC6526815
- DOI: 10.1080/15548627.2019.1569933
VCP maintains lysosomal homeostasis and TFEB activity in differentiated skeletal muscle
Abstract
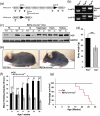
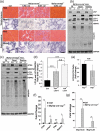
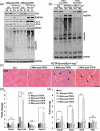
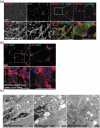
Differentiated tissue is particularly vulnerable to alterations in protein and organelle homeostasis. The essential protein VCP, mutated in hereditary inclusion body myopathy, amyotrophic lateral sclerosis and frontotemporal dementia, is critical for efficient clearance of misfolded proteins and damaged organelles in dividing cells, but its role in terminally differentiated tissue affected by disease mutations is less clear. To understand the relevance of VCP in differentiated tissue, we inactivated it in skeletal muscle of adult mice. Surprisingly, knockout muscle demonstrated a necrotic myopathy with increased macroautophagic/autophagic proteins and damaged lysosomes. This was not solely due to a defect in autophagic degradation because age-matched mice with muscle inactivation of the autophagy essential protein, ATG5, did not demonstrate a myopathy. Notably, myofiber necrosis was preceded by upregulation of LGALS3/Galectin-3, a marker of damaged lysosomes, and TFEB activation, suggesting early defects in the lysosomal system. Consistent with that, myofiber necrosis was recapitulated by chemical induction of lysosomal membrane permeabilization (LMP) in skeletal muscle. Moreover, TFEB was activated after LMP in cells, but activation and nuclear localization of TFEB persisted upon VCP inactivation or disease mutant expression. Our data identifies VCP as central mediator of both lysosomal clearance and biogenesis in skeletal muscle. Abbreviations: AAA: ATPases Associated with diverse cellular Activities; TUBA1A/α-tubulin: tubulin alpha 1a; ATG5: autophagy related 5; ATG7: autophagy related 7; ACTA1: actin alpha 1, skeletal muscle; CLEAR: coordinated lysosomal expression and regulation; CTSB/D: cathepsin B/D; Ctrl: control; DAPI: diamidino-2-phenylindole; EBSS: Earle's balanced salt solution; ELDR: endolysosomal damage response; ESCRT: endosomal sorting complexes required for transport; Gastroc/G: gastrocnemius; H&E: hematoxylin and eosin; HSPA5/GRP78: heat shock protein family A (Hsp70) member 5; IBMPFD/ALS: inclusion body myopathy associated with Paget disease of the bone, frontotemporal dementia and amyotrophic lateral sclerosis; i.p.: intraperitoneal; LAMP1/2: lysosomal-associated membrane protein 1/2; LLOMe: Leu-Leu methyl ester hydrobromide; LGALS3/Gal3: galectin 3; LMP: lysosomal membrane permeabilization; MTOR: mechanistic target of rapamycin kinase; MYL1: myosin light chain 1; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MSP: multisystem proteinopathy; PBS: phosphate-buffered saline; PCR: polymerase chain reaction; Quad/Q: quadriceps; RHEB: Ras homolog, mTORC1 binding; SQSTM1: sequestosome 1; TFEB: transcription factor EB; TA: tibialis anterior; siRNA: small interfering RNA; SQSTM1/p62, sequestosome 1; TARDBP/TDP-43: TAR DNA binding protein; TBS: Tris-buffered saline; TXFN, tamoxifen; UBXN6/UBXD1: UBX domain protein 6; VCP: valosin containing protein; WT: wild-type.
Keywords: Autophagy; TFEB; VCP; lysosome; myopathy; skeletal muscle.
Figures
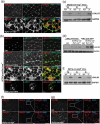

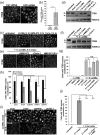


References
-
- Lan B, Chai S, Wang P, et al. VCP/p97/Cdc48, a linking of protein homeostasis and cancer therapy. Curr Mol Med. 2018. March 7;17:608–618. PubMed PMID: 29521227. - PubMed
-
- Muller JM, Deinhardt K, Rosewell I, et al. Targeted deletion of p97 (VCP/CDC48) in mouse results in early embryonic lethality. Biochem Biophys Res Commun. 2007. March 9;354(2):459–465. PubMed PMID: 17239345. - PubMed
-
- Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004. April;36(4):377–381. PubMed PMID: 15034582. - PubMed
-
- Taylor JP. Multisystem proteinopathy: intersecting genetics in muscle, bone, and brain degeneration. Neurology. 2015. August 25;85(8):658–660. PubMed PMID: 26208960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
