Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats
- PMID: 30654793
- PMCID: PMC6337851
- DOI: 10.1186/s12906-018-2427-y
Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats
Abstract
Background: Impaired wound healing is a debilitating complication of diabetes that leads to significant morbidity, particularly foot ulcers. The risk of developing diabetic foot ulcers for diabetic patients is 15% over their lifetime and approximately 85% of limb amputations is caused by non-healing ulcers. Unhealed, gangrenous wounds destroy the structural integrity of the skin, which acts as a protective barrier that prevents the invasion of external noxious agents into the body. Vicenin-2 (VCN-2) has been reported to contain prospective anti-oxidant and anti-inflammatory properties that enhance cell proliferation and migration. Sodium Alginate (SA) is a natural polysaccharide that possesses gel forming properties and has biodegradable and biocompatible characteristics. Therefore, the objective of this study is to evaluate the effect of SA wound dressings containing VCN-2 on diabetic wounds.
Methods: Wounds were inflicted in type-1 diabetic-streptozotocin (STZ) induced male Sprague Dawley rats. Subsequently, relevant groups were topically treated with the indicated concentrations (12.5, 25 and 50 μM) of VCN-2 hydrocolloid film over the study duration (14 days). The control group was treated with vehicle dressing (blank or allantoin). Wounded tissues and blood serum were collected on 0, 7 and 14 days prior to sacrifice. Appropriate wound assessments such as histological tests, nitric oxide assays, enzyme-linked immunosorbent assays (ELISA) and immunoblotting assays were conducted to confirm wound healing efficacy in the in vivo model. One-way Analysis of Variance (ANOVA) was used for statistical analysis.
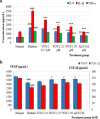
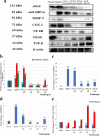
Results: Results showed that hydrocolloid film was recapitulated with VCN-2 enhanced diabetic wound healing in a dose-dependent manner. VCN-2 reduced pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), mediators (iNOS and COX-2), and nitric oxide (NO) via the NF-κB pathway. Data suggests that the VCN-2 film facilitated healing in hyperglycemic conditions by releasing growth factors such as (VEGF and TGF-β) to enhance cell proliferation, migration, and wound contraction via the VEGF and TGF-β mechanism pathways.
Conclusions: This study's findings suggest that VCN-2 may possess wound healing potential since topical treatment with VCN-2 hydrocolloid films effectively enhanced wound healing in hyperglycemic conditions.
Keywords: Diabetic wound; Hydrocolloid film; Sodium alginate; Vicenin-2.
Conflict of interest statement
Ethics approval
Institutional Animal Care and Use Committee (IACUC) of the Universiti Putra Malaysia (authorization number UPM/IACUC/AUP-R079/2016) approved this animal study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

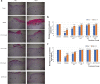

 : angiogenesis and
: angiogenesis and  : fibroblast cell. b Histological finding score of fibroblast proliferation; (c) angiogenesis and (d) inflammatory cells infiltrate (score from 0 to 4) from 10 randomly chosen high-power fields (× 400) from three sections in wound treated with difference films on day 7 and 14. Data are means ± SEM histological score (n = 6). ###p < 0.001, diabetes versus normal; *p < 0.05, **p < 0.01 and ***p < 0.001, treatment groups versus diabetic
: fibroblast cell. b Histological finding score of fibroblast proliferation; (c) angiogenesis and (d) inflammatory cells infiltrate (score from 0 to 4) from 10 randomly chosen high-power fields (× 400) from three sections in wound treated with difference films on day 7 and 14. Data are means ± SEM histological score (n = 6). ###p < 0.001, diabetes versus normal; *p < 0.05, **p < 0.01 and ***p < 0.001, treatment groups versus diabetic

References
-
- Thu HE, Zulfakar MH, Ng SF. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int J Pharm. 2012;434(2):375–383. - PubMed
-
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. - PubMed
-
- Declue CE, Shornick LP. The cytokine milieu of diabetic wounds. Diabetes Management. 2015;5(6):525–537.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

