Susceptibility of Plasmodium falciparum to artemisinins and Plasmodium vivax to chloroquine in Phuoc Chien Commune, Ninh Thuan Province, south-central Vietnam
- PMID: 30654808
- PMCID: PMC6335800
- DOI: 10.1186/s12936-019-2640-2
Susceptibility of Plasmodium falciparum to artemisinins and Plasmodium vivax to chloroquine in Phuoc Chien Commune, Ninh Thuan Province, south-central Vietnam
Abstract
Background: Reduced artemisinin susceptibility and artemisinin-based combination therapy (ACT)-resistance against Plasmodium falciparum and chloroquine (CQ)-resistant P. vivax malaria has been reported in Vietnam. Two therapeutic efficacy studies were conducted in Thuan Bac District (Ninh Thuan Province, Vietnam) in 2015 and 2016 to determine the extent of reduced artemisinin susceptibility and ACT resistant falciparum malaria, and CQ-resistant vivax malaria were present.
Methods: Twenty-seven patients with falciparum malaria were randomized to receive artesunate alone (AS ~ 4 mg/kg/day) for 4 days followed by dihydroartemisinin (DHA) (2.2 mg/kg)-piperaquine (PPQ) (18 mg/kg) daily for 3 days or artemether (AM) (1.7 mg/kg)-lumefantrine (LUM) (12 mg/kg) twice daily for 3 days. Sixteen subjects with vivax malaria received CQ (total 25 mg/kg over 3 days). The therapeutic efficacy study for treating falciparum malaria was complemented with molecular analysis for artemisinin and piperaquine resistance, and in vitro drug susceptibility testing. Patient's drug exposure following both falciparum and vivax treatment studies was determined.
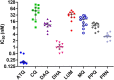
Results: Twenty-five of 27 patients treated with the artemisinin regimens completed the 42-day follow-up period. None had parasites present on day 3 after commencing treatment with no incidence of recrudescence (100% curative rate). One patient on AS + DHA-PPQ was lost to follow-up and one patient had Plasmodium falciparum and Plasmodium vivax infection on day 0 by PCR. Of the vivax patients, 15 of 16 completed CQ treatment and two had a recurrence of vivax malaria on day 28, a failure rate of 13.3% (2/15). No mutations in the Pfkelch-13 gene for artemisinin resistance or exo-E415G gene polymorphism and amplification in plasmepsins 2 and 3 for piperaquine resistance were observed. In vitro testing of patient's falciparum parasites indicated susceptibility (low IC50 nM values) to dihydroartemisinin, lumefantrine, piperaquine and pyronaridine. Patient's drug exposure to artesunate and lumefantrine was comparable to published data, however, blood CQ concentrations were lower.
Conclusions: Clinical findings, molecular analysis and in vitro testing revealed that the falciparum parasites at Phuoc Chien Commune were artemisinin susceptible. The clinical failure rate of the 15 vivax patients who completed CQ treatment was 13%. Further studies are required to determine whether CQ-resistant vivax malaria is present at the commune.
Keywords: Artemether; Artesunate; Chloroquine; Dihydroartemisinin; In vitro drug susceptibility testing; Lumefantrine; Malaria; Pfkelch-13; Piperaquine; Plasmodium falciparum; Plasmodium vivax; Vietnam.
Figures
Similar articles
-
Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria in Papua New Guinea.Malar J. 2018 Oct 5;17(1):350. doi: 10.1186/s12936-018-2494-z. Malar J. 2018. PMID: 30290825 Free PMC article. Clinical Trial.
-
Therapeutic efficacies of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum and chloroquine and dihydroartemisinin-piperaquine for uncomplicated Plasmodium vivax infection in Ethiopia.Malar J. 2022 Dec 1;21(1):359. doi: 10.1186/s12936-022-04350-z. Malar J. 2022. PMID: 36451216 Free PMC article. Clinical Trial.
-
A trial of combination antimalarial therapies in children from Papua New Guinea.N Engl J Med. 2008 Dec 11;359(24):2545-57. doi: 10.1056/NEJMoa0804915. Epub 2008 Dec 8. N Engl J Med. 2008. PMID: 19064624 Clinical Trial.
-
Monitoring antimalarial drug efficacy in the Greater Mekong Subregion: an overview of in vivo results from 2008 to 2010.Southeast Asian J Trop Med Public Health. 2013;44 Suppl 1:201-30; discussion 306-7. Southeast Asian J Trop Med Public Health. 2013. PMID: 24159833 Review.
-
Risk of Plasmodium vivax parasitaemia after Plasmodium falciparum infection: a systematic review and meta-analysis.Lancet Infect Dis. 2019 Jan;19(1):91-101. doi: 10.1016/S1473-3099(18)30596-6. Lancet Infect Dis. 2019. PMID: 30587297 Free PMC article.
Cited by
-
Efficacy of dihydroartemisinin/piperaquine and artesunate monotherapy for the treatment of uncomplicated Plasmodium falciparum malaria in Central Vietnam.J Antimicrob Chemother. 2020 Aug 1;75(8):2272-2281. doi: 10.1093/jac/dkaa172. J Antimicrob Chemother. 2020. PMID: 32437557 Free PMC article.
-
Molecular surveillance and temporal monitoring of malaria parasites in focal Vietnamese provinces.Malar J. 2020 Dec 31;19(1):458. doi: 10.1186/s12936-020-03561-6. Malar J. 2020. PMID: 33384023 Free PMC article.
-
Magnitude and patterns of severe Plasmodium vivax monoinfection in Vietnam: a 4-year single-center retrospective study.Front Med (Lausanne). 2023 May 30;10:1128981. doi: 10.3389/fmed.2023.1128981. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37324161 Free PMC article.
-
Microhaplotype deep sequencing assays to capture Plasmodium vivax infection lineages.Nat Commun. 2025 Aug 5;16(1):7192. doi: 10.1038/s41467-025-62357-x. Nat Commun. 2025. PMID: 40764298 Free PMC article. Clinical Trial.
-
A single point mutation in the Plasmodium falciparum 3'-5' exonuclease does not alter piperaquine susceptibility.Malar J. 2022 Apr 22;21(1):130. doi: 10.1186/s12936-022-04148-z. Malar J. 2022. PMID: 35459163 Free PMC article.
References
-
- WHO . World malaria report 2017. Geneva: World Health Organization; 2017.
-
- Saunders D, Vanachayangkul P, Lon C, U.S. Army Military Malaria Research Program. National Center for Parasitology, Entomology, and Malaria Control (CNM) Royal Cambodian Armed Forces Dihydroartemisinin–piperaquine failure in Cambodia. N Engl J Med. 2014;371:484–485. doi: 10.1056/NEJMc1403007. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous