The prolonged interval between induction chemotherapy and radiotherapy is associated with poor prognosis in patients with nasopharyngeal carcinoma
- PMID: 30654815
- PMCID: PMC6335732
- DOI: 10.1186/s13014-019-1213-4
The prolonged interval between induction chemotherapy and radiotherapy is associated with poor prognosis in patients with nasopharyngeal carcinoma
Abstract
Objectives: Induction chemotherapy (IC) now is gaining recognition for the treatment of nasopharyngeal carcinoma (NPC). The current study was conducted to examine the association between prognosis and the interval between IC and radiotherapy (RT) in NPC patients.
Methods: Patients with newly diagnosed, non-metastatic NPC who were treated with IC followed by RT from 2009 to 2012 were identified from an inpatient database. Overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS) and locoregional recurrence-free survival (LRFS) were compared between those with interval ≤ 30 and > 30 days by Kaplan-Meier and log-rank analyses; Cox modeling was used for multivariable analysis.
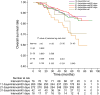
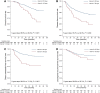
Results: A total of 668 patients met inclusion criteria with median follow-up of 64.4 months. Patients were categorized by interval: 608 patients with interval ≤ 30 days, and 60 with interval > 30 days. The 5-year OS, DFS, DMFS and LRFS rates were 86.6, 78.2, 88.0 and 89.8% for patients with interval ≤ 30 days, respectively, and 69.2, 64.5, 71.2 and 85.1% for patients with interval > 30 days, respectively. The prolonged interval was a risk factor for OS, DFS and DMFS with adjusted hazard ratios (95% confidence intervals) were 2.44 (1.48-4.01), 1.99 (1.27-3.11) and 2.62 (1.54-4.47), respectively.
Conclusions: Prolonged interval > 30 days was associated with a significantly higher risk of distant metastasis and death in NPC patients. Efforts should be made to avoid prolonged interval between IC and RT to minimize the risk of treatment failure.
Keywords: Induction chemotherapy; Interval; Nasopharyngeal carcinoma; Prognosis; Radiotherapy.
Conflict of interest statement
Ethics approval and consent to participate
The clinical research ethics committee of Sun Yat-sen University Cancer Center approved this study. As this was a retrospective analysis of routine data, we were granted a waiver for written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
An open-label, single-arm phase II clinical study of induction chemotherapy and sequential Nimotuzumab combined with concurrent chemoradiotherapy in N3M0 stage nasopharyngeal carcinoma.J BUON. 2018 Nov-Dec;23(6):1656-1661. J BUON. 2018. PMID: 30610791 Clinical Trial.
-
Non-metastatic stage IV nasopharyngeal carcinoma patients: analysis of the pattern of relapse and survival.Radiother Oncol. 2004 Jul;72(1):71-7. doi: 10.1016/j.radonc.2004.02.012. Radiother Oncol. 2004. PMID: 15236877
-
Induction chemotherapy followed by concurrent chemoradiotherapy vs. concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a retrospective cohort study.Cancer Chemother Pharmacol. 2017 Jun;79(6):1087-1097. doi: 10.1007/s00280-017-3297-6. Epub 2017 Apr 20. Cancer Chemother Pharmacol. 2017. PMID: 28429051
-
The Tumour Response to Induction Chemotherapy has Prognostic Value for Long-Term Survival Outcomes after Intensity-Modulated Radiation Therapy in Nasopharyngeal Carcinoma.Sci Rep. 2016 Apr 21;6:24835. doi: 10.1038/srep24835. Sci Rep. 2016. PMID: 27099096 Free PMC article.
-
Recombinant human adenovirus-p53 therapy for the treatment of nasopharyngeal carcinoma: a meta-analysis.Springerplus. 2016 Oct 27;5(1):1885. doi: 10.1186/s40064-016-3574-6. eCollection 2016. Springerplus. 2016. PMID: 27843742 Free PMC article. Review.
Cited by
-
Treatment of Childhood Nasopharyngeal Carcinoma With Induction Chemotherapy and Concurrent Chemoradiotherapy: Results of the Children's Oncology Group ARAR0331 Study.J Clin Oncol. 2019 Dec 10;37(35):3369-3376. doi: 10.1200/JCO.19.01276. Epub 2019 Sep 25. J Clin Oncol. 2019. PMID: 31553639 Free PMC article.
-
The Most Efficacious Induction Chemotherapy Regimen for Locoregionally Advanced Nasopharyngeal Carcinoma: A Network Meta-Analysis.Front Oncol. 2021 Feb 25;11:626145. doi: 10.3389/fonc.2021.626145. eCollection 2021. Front Oncol. 2021. PMID: 33718193 Free PMC article.
-
Improved overall survival is associated with adjuvant chemotherapy after definitive concurrent chemoradiotherapy for N3 nasopharyngeal cancer.Sci Rep. 2022 Aug 4;12(1):13390. doi: 10.1038/s41598-022-16422-w. Sci Rep. 2022. PMID: 35927415 Free PMC article.
-
Identification of DTL as Related Biomarker and Immune Infiltration Characteristics of Nasopharyngeal Carcinoma via Comprehensive Strategies.Int J Gen Med. 2022 Mar 2;15:2329-2345. doi: 10.2147/IJGM.S352330. eCollection 2022. Int J Gen Med. 2022. PMID: 35264872 Free PMC article.
-
Evaluation of Genes and Molecular Pathways Involved in Pathogenesis of Sickle Cell Anemia: A Bioinformatics Analysis and Future Perspective.Iran J Public Health. 2024 Jun;53(6):1404-1415. Iran J Public Health. 2024. PMID: 39430157 Free PMC article.
References
-
- Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–668. doi: 10.1016/j.ijrobp.2010.03.024. - DOI - PubMed
MeSH terms
Grants and funding
- 2017A030312003/Natural Science Foundation of Guang Dong Province
- 201803040003/Health & Medical Collaborative Innovation Project of Guangzhou City, China
- IRT_17R110/Innovation Team Development Plan of the Ministry of Education
- B14035/Overseas Expertise Introduction Project for Discipline Innovation (111 Project)
LinkOut - more resources
Full Text Sources
Miscellaneous

