Combinatorial approach for improved cyanidin 3-O-glucoside production in Escherichia coli
- PMID: 30654816
- PMCID: PMC6335687
- DOI: 10.1186/s12934-019-1056-6
Combinatorial approach for improved cyanidin 3-O-glucoside production in Escherichia coli
Abstract
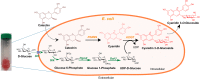
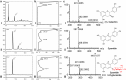
Background: Multi-monocistronic and multi-variate vectors were designed, built, and tested for the improved production of cyanidin 3-O-glucoside (C3G) in Escherichia coli BL21 (DE3). The synthetic bio-parts were designed in such a way that multiple genes can be assembled using the bio-brick system, and expressed under different promoters in a single vector. The vectors harbor compatible cloning sites, so that the genes can be shuffled from one vector to another in a single step, and assembled into a single vector. The two required genes: anthocyanidin synthase (PhANS) from Petunia hybrida, and cyanidin 3-O-glucosyltransferase (At3GT) from Arabidopsis thaliana, were individually cloned under PT7, Ptrc, and PlacUV5 promoters. Both PhANS and At3GT were shuffled back and forth, so as to generate a combinatorial system for C3G production. The constructed systems were further coupled with the genes for UDP-D-glucose synthesis, all cloned in a multi-monocistronic fashion under PT7. Finally, the production of C3G was checked and confirmed using the modified M9 media, and analyzed through various chromatography and spectrometric analyses.
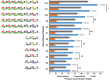
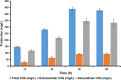
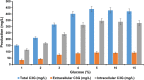
Results: The engineered strains endowed with newly generated vectors and the genes for C3G biosynthesis and UDP-D-glucose synthesis were fed with 2 mM (+)-catechin and D-glucose for the production of cyanidin, and its subsequent conversion to C3G. One of the engineered strains harboring At3GT and PhANS under Ptrc promoter and UDP-D-glucose biosynthesis genes under PT7 promoter led to the production of ~ 439 mg/L of C3G within 36 h of incubation, when the system was exogenously fed with 5% (w/v) D-glucose. This system did not require exogenous supplementation of UDP-D-glucose.
Conclusion: A synthetic vector system using different promoters has been developed and used for the synthesis of C3G in E. coli BL21 (DE3) by directing the metabolic flux towards the UDP-D-glucose. This system has the potential of generating better strains for the synthesis of valuable natural products.
Keywords: Anthocyanin; Cyanidin 3-O-glucoside; Multi-monocistronic; UDP-D-glucose.
Figures


References
-
- Gündüz K, Serçe S, Hancock JF. Variation among highbush and rabbit eye cultivars of blueberry for fruit quality and phytochemical characteristics. J Food Compos Anal. 2015;38:69–79. doi: 10.1016/j.jfca.2014.09.007. - DOI
-
- Giampieri F, Alvarez-Suarez JM, Mazzoni L, Forbes-Hernandez TY, Gasparrini M, Gonzalez-Paramas AM, Santos-Buelga C, Quiles JL, Bompadre S, Mezzetti B, Battino M. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014;5:1939. doi: 10.1039/C4FO00048J. - DOI - PubMed
-
- Klimis-Zacas D, Vendrame S, Kristo AS. Wild blueberries attenuate risk factors of the metabolic syndrome. J Berry Res. 2016;6:225–236. doi: 10.3233/JBR-160136. - DOI
-
- Rodriguez-Mateos A, Feliciano RP, Cifuentes-Gomez T, Spencer JP. Bioavailability of wild blueberry (poly) phenols at different levels of intake. J Berry Res. 2016;6:137–148. doi: 10.3233/JBR-160123. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

