Temporary transvenous diaphragm pacing vs. standard of care for weaning from mechanical ventilation: study protocol for a randomized trial
- PMID: 30654837
- PMCID: PMC6337771
- DOI: 10.1186/s13063-018-3171-9
Temporary transvenous diaphragm pacing vs. standard of care for weaning from mechanical ventilation: study protocol for a randomized trial
Abstract
Background: Mechanical ventilation (MV) is a life-saving technology that restores or assists breathing. Like any treatment, MV has side effects. In some patients it can cause diaphragmatic atrophy, injury, and dysfunction (ventilator-induced diaphragmatic dysfunction, VIDD). Accumulating evidence suggests that VIDD makes weaning from MV difficult, which involves increased morbidity and mortality.
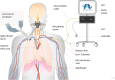
Methods and analysis: This paper describes the protocol of a randomized, controlled, open-label, multicenter trial that is designed to investigate the safety and effectiveness of a novel therapy, temporary transvenous diaphragm pacing (TTVDP), to improve weaning from MV in up to 88 mechanically ventilated adult patients who have failed at least two spontaneous breathing trials over at least 7 days. Patients will be randomized (1:1) to TTVDP (treatment) or standard of care (control) groups. The primary efficacy endpoint is time to successful extubation with no reintubation within 48 h. Secondary endpoints include maximal inspiratory pressure and ultrasound-measured changes in diaphragm thickness and diaphragm thickening fraction over time. In addition, observational data will be collected and analyzed, including 30-day mortality and time to discharge from the intensive care unit and from the hospital. The hypothesis to be tested postulates that more TTVDP patients than control patients will be successfully weaned from MV within the 30 days following randomization.
Discussion: This study is the first large-scale clinical trial of a novel technology (TTVDP) aimed at accelerating difficult weaning from MV. The technology tested provides the first therapy directed specifically at VIDD, an important cause of delayed weaning from MV. Its results will help delineate the place of this therapeutic approach in clinical practice and help design future studies aimed at defining the indications and benefits of TTVDP.
Trial registration: ClinicalTrials.gov, NCT03096639 . Registered on 30 March 2017.
Keywords: Diaphragm; Mechanical ventilation; Phrenic stimulation; Ventilator-induced diaphragmatic dysfunction; Weaning.
Conflict of interest statement
Ethics approval and consent to participate
The RESCUE 2 study has been ethically approved by competent institutional bodies in the two participating countries. In France, a global authorization relevant for all study sites has been granted by the Comité de Protection des Personnes Sud-Est VI, Clermont-Ferrand, France (decision # dated November 9, 2017) and by the competent authority (Agence Nationale de Sécurité du Médicament et des produits de santé) on June 32,017. In Germany, the study has been approved by the central Ethics Committee RWTH Aachen (August 14, 2017), which is relevant for each participating center and the competent authority (Bundesinstitut fur Arzneimittel und Medizinprodukte (BfArM) on September 1, 2017. Written informed consent will be obtained from all patients participating in the study, as a mandatory prerequisite to inclusion.
Consent for publication
Not applicable.
Competing interests
DE reports personal fees from Lungpacer Medical Inc. during the conduct of the study and outside the submitted work. In addition, DE has multiple related patents issued and pending. DS reports personal fees from Lungpacer Medical Inc. during the conduct of the study. LC reports fees from Lungpacer Medical Inc. during the conduct of the study and other competing interests from Lungpacer Medical Inc. outside the submitted work. GJC reports grants from Boehringer Ingelheim, Novartis, AstraZeneca, Respironics, MedImmune, Actelion, Forest, Pearl, Ikaria, Aeris, PneumRx, and Pulmonx and other support from HGE Health Care Solutions, LLC, Almirall, Boehringer Ingelheim, and Holaira, all outside the submitted work. MD reports personal fees and non-financial support from Lungpacer Medical Inc. during the conduct of the study and personal fees and non-financial support from Pulsion Medical Systems outside the submitted work. MGA reports personal fees from Lungpacer Medical Inc. during the conduct of the study and grants and personal fees from Dräger Medical AG, GlaxoSmithKline (GSK), and GE Healthcare outside the submitted work. DMD reports other support as well as research funding and consulting fees from Lungpacer Medical Inc. during the conduct of the study. BJP reports personal fees from Lungpacer Medical Inc. during the conduct of the study. TN reports personal fees from Lungpacer Medical Inc. during the conduct of the study and personal fees from Lungpacer Medical Inc. outside the submitted work. TS reports personal fees from Lungpacer Medical Inc. during the conduct of the study and personal fees from AstraZeneca France, personal fees from Boehringer Ingelheim France, personal fees from GSK France, personal fees and non-financial support from Novartis France, personal fees from TEVA France, personal fees from Chiesi, personal fees from Pierre Fabre Médicament, and personal fees from Invacare France, outside the submitted work. In the domain of therapeutic phrenic stimulation, TS received honoraria from Synapse Biomedical to translate the DPS/NeurRx4 user’s manual from English to French in 2007, and from 2012 to 2016, Synapse Biomedical contributed to a fundraiser organized by TS to promote respiratory research. TS advised Synapse Biomedical about clinical protocols but did not receive any honoraria from this company. TS currently collaborates with another diaphragm pacing company (Neuroresp/Atrotech) in an advisory capacity, here also without any honoraria or any non-financial support. FL declares that he has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U.S. hospitals, 2011: Statistical Brief #165. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville: Agency for Healthcare Research and Quality; 2013. - PubMed
-
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370(10):980. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

