Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review
- PMID: 30654892
- PMCID: PMC6865825
- DOI: 10.1016/j.jacc.2018.09.089
Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review
Abstract
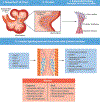
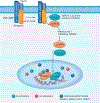
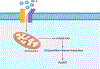
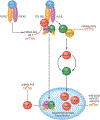
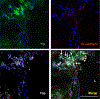
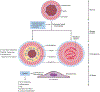
Endothelial to mesenchymal transition (EndMT) is a process whereby an endothelial cell undergoes a series of molecular events that lead to a change in phenotype toward a mesenchymal cell (e.g., myofibroblast, smooth muscle cell). EndMT plays a fundamental role during development, and mounting evidence indicates that EndMT is involved in adult cardiovascular diseases (CVDs), including atherosclerosis, pulmonary hypertension, valvular disease, and fibroelastosis. Therefore, the targeting of EndMT may hold therapeutic promise for treating CVD. However, the field faces a number of challenges, including the lack of a precise functional and molecular definition, a lack of understanding of the causative pathological role of EndMT in CVDs (versus being a "bystander-phenomenon"), and a lack of robust human data corroborating the extent and causality of EndMT in adult CVDs. Here, we review this emerging but exciting field, and propose a framework for its systematic advancement at the molecular and translational levels.
Keywords: EndMT; cardiovascular; endothelial to mesenchymal transition.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hamburger V Introduction: Johannes Holtfreter, pioneer in experimental embryology. Dev Dyn 1996;205:214–6. - PubMed
-
- Trelstad RL, Hay ED, Revel JD. Cell contact during early morphogenesis in the chick embryo. Dev Biol 1967;16:78–106. - PubMed
-
- Grande MT, Sanchez-Laorden B, Lopez-Blau C et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 2015;21:989–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

