Quantitative 3D Mapping of the Human Skeletal Muscle Mitochondrial Network
- PMID: 30655224
- PMCID: PMC6513570
- DOI: 10.1016/j.celrep.2019.01.010
Quantitative 3D Mapping of the Human Skeletal Muscle Mitochondrial Network
Erratum in
-
Quantitative 3D Mapping of the Human Skeletal Muscle Mitochondrial Network.Cell Rep. 2019 Apr 2;27(1):321. doi: 10.1016/j.celrep.2019.03.051. Cell Rep. 2019. PMID: 30943412 Free PMC article. No abstract available.
Abstract
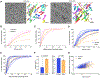
Genetic and biochemical defects of mitochondrial function are a major cause of human disease, but their link to mitochondrial morphology in situ has not been defined. Here, we develop a quantitative three-dimensional approach to map mitochondrial network organization in human muscle at electron microscopy resolution. We establish morphological differences between human and mouse and among patients with mitochondrial DNA (mtDNA) diseases compared to healthy controls. We also define the ultrastructure and prevalence of mitochondrial nanotunnels, which exist as either free-ended or connecting membrane protrusions across non-adjacent mitochondria. A multivariate model integrating mitochondrial volume, morphological complexity, and branching anisotropy computed across individual mitochondria and mitochondrial populations identifies increased proportion of simple mitochondria and nanotunnels as a discriminant signature of mitochondrial stress. Overall, these data define the nature of the mitochondrial network in human muscle, quantify human-mouse differences, and suggest potential morphological markers of mitochondrial dysfunction in human tissues.
Keywords: 3D morphometry; imaging; machine learning; mitochondrial disease; mitochondrion; nanotunnel; reconstruction; reticulum; serial block-face SEM; skeletal muscle.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


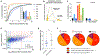


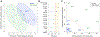
References
-
- Bakeeva LE, Chentsov YuS, and Skulachev VP (1978). Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim. Bio-phys. Acta 501, 349–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

