CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors
- PMID: 30655315
- PMCID: PMC8456711
- DOI: 10.1158/1078-0432.CCR-18-0432
CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors
Abstract
Purpose: Patients with relapsed pediatric solid tumors and CNS malignancies have few therapeutic options and frequently die of their disease. Chimeric antigen receptor (CAR) T cells have shown tremendous success in treating relapsed pediatric acute lymphoblastic leukemia, but this has not yet translated to treating solid tumors. This is partially due to a paucity of differentially expressed cell surface molecules on solid tumors that can be safely targeted. Here, we present B7-H3 (CD276) as a putative target for CAR T-cell therapy of pediatric solid tumors, including those arising in the central nervous system.
Experimental design: We developed a novel B7-H3 CAR whose binder is derived from a mAb that has been shown to preferentially bind tumor tissues and has been safely used in humans in early-phase clinical trials. We tested B7-H3 CAR T cells in a variety of pediatric cancer models.
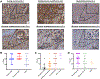
Results: B7-H3 CAR T cells mediate significant antitumor activity in vivo, causing regression of established solid tumors in xenograft models including osteosarcoma, medulloblastoma, and Ewing sarcoma. We demonstrate that B7-H3 CAR T-cell efficacy is largely dependent upon high surface target antigen density on tumor tissues and that activity is greatly diminished against target cells that express low levels of antigen, thus providing a possible therapeutic window despite low-level normal tissue expression of B7-H3.
Conclusions: B7-H3 CAR T cells could represent an exciting therapeutic option for patients with certain lethal relapsed or refractory pediatric malignancies, and should be tested in carefully designed clinical trials.
©2019 American Association for Cancer Research.
Figures





References
-
- Rodriguez-Galindo C, Billups CA, Kun LE, Rao BN, Pratt CB, Merchant TE, et al.Survival after recurrence of Ewing tumors: the St Jude Children’s Research Hospital experience, 1979–1999. Cancer 2002;94:561–9. - PubMed
-
- Tomlinson FH, Scheithauer BW, Meyer FB, Smithson WA, Shaw EG, Miller GM, et al.Medulloblastoma: I. Clinical, diagnostic, and therapeutic overview. J Child Neurol 1992;7:142–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

