In-vivo correlations between skin metabolic oscillations and vasomotion in wild-type mice and in a model of oxidative stress
- PMID: 30655574
- PMCID: PMC6336806
- DOI: 10.1038/s41598-018-36970-4
In-vivo correlations between skin metabolic oscillations and vasomotion in wild-type mice and in a model of oxidative stress
Abstract
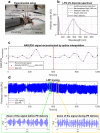
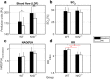
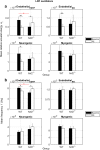
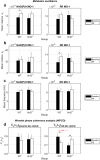
Arterioles in the cutaneous microcirculation frequently display an oscillatory phenomenon defined vasomotion, consistent with periodic diameter variations in the micro-vessels associated with particular physiological or abnormal conditions. The cellular mechanisms underlying vasomotion and its physiological role have not been completely elucidated. Various mechanisms were demonstrated, based on cell Ca2+ oscillations determined by the activity of channels in the plasma membrane or sarcoplasmic reticulum of vascular cells. However, the possible engagement in vasomotion of cell metabolic oscillations of mitochondrial or glycolytic origin has been poorly explored. Metabolic oscillations associated with the production of ATP energy were previously described in cells, while limited studies have investigated these fluctuations in-vivo. Here, we characterised a low-frequency metabolic oscillator (MO-1) in skin from live wild-type and Nrf2-/- mice, by combination of fluorescence spectroscopy and wavelet transform processing technique. Furthermore, the relationships between metabolic and microvascular oscillators were examined during phenylephrine-induced vasoconstriction. We found a significant interaction between MO-1 and the endothelial EDHF vasomotor mechanism that was reduced in the presence of oxidative stress (Nrf2-/- mice). Our findings suggest indirectly that metabolic oscillations may be involved in the mechanisms underlying endothelium-mediated skin vasomotion, which might be altered in the presence of metabolic disturbance.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Longchain n-3 polyunsaturated fatty acids and microvascular reactivity: observation in the hamster cheek pouch.Microvasc Res. 2007 May;73(3):237-47. doi: 10.1016/j.mvr.2006.11.002. Epub 2006 Dec 28. Microvasc Res. 2007. PMID: 17196224
-
Vasomotion and underlying mechanisms in small arteries. An in vitro study of rat blood vessels.Acta Physiol Scand Suppl. 1993;614:1-44. Acta Physiol Scand Suppl. 1993. PMID: 8128886 Review.
-
Poiseuille, periodicity, and perfusion: rhythmic oscillatory vasomotion in the skin.J Invest Dermatol. 1989 Aug;93(2 Suppl):113S-118S. doi: 10.1111/1523-1747.ep12581224. J Invest Dermatol. 1989. PMID: 2502581
-
Activation of thromboxane receptors and the induction of vasomotion in the hamster cheek pouch microcirculation.Br J Pharmacol. 1997 Nov;122(5):859-66. doi: 10.1038/sj.bjp.0701464. Br J Pharmacol. 1997. PMID: 9384501 Free PMC article.
-
Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion.Dan Med Bull. 2010 Oct;57(10):B4191. Dan Med Bull. 2010. PMID: 21040688 Review.
Cited by
-
Study of the Synchronization and Transmission of Intracellular Signaling Oscillations in Cells Using Bispectral Analysis.Biology (Basel). 2024 Sep 2;13(9):685. doi: 10.3390/biology13090685. Biology (Basel). 2024. PMID: 39336112 Free PMC article.
-
Strain- and sex-specific differences in intestinal microhemodynamics and gut microbiota composition.Gastroenterol Rep (Oxf). 2024 Sep 16;12:goae087. doi: 10.1093/gastro/goae087. eCollection 2024. Gastroenterol Rep (Oxf). 2024. PMID: 39286773 Free PMC article.
-
Sural Nerve Perfusion in Mice.Front Neurosci. 2020 Dec 10;14:579373. doi: 10.3389/fnins.2020.579373. eCollection 2020. Front Neurosci. 2020. PMID: 33362454 Free PMC article.
References
-
- Traikov L, Ushiyama A, Lawlor G, Sasaki R, Ohkubo C. Subcutaneous arteriolar vasomotion changes during and after ELF-EMF exposure in mice in vivo. Environmentalis. 2005;25(2–4):93–101. doi: 10.1007/s10669-005-4271-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

