Approaches to urinary detection of prostate cancer
- PMID: 30655600
- PMCID: PMC6640078
- DOI: 10.1038/s41391-019-0127-4
Approaches to urinary detection of prostate cancer
Abstract
Background: Prostate cancer is the most common cancer in American men that ranges from low risk states amenable to active surveillance to high-risk states that can be lethal especially if untreated. There is a critical need to develop relatively non-invasive and clinically useful methods for screening, detection, prognosis, disease monitoring, and prediction of treatment efficacy. In this review, we focus on important advances as well as future efforts needed to drive clinical innovation in this area of urine biomarker research for prostate cancer detection and prognostication.
Methods: We provide a review of current literature on urinary biomarkers for prostate cancer. We evaluate the strengths and limitations of a variety of approaches that vary in sampling strategies and targets measured; discuss reported urine tests for prostate cancer with respect to their technical, analytical, and clinical parameters; and provide our perspectives on critical considerations in approaches to developing a urine-based test for prostate cancer.
Results: There has been an extensive history of exploring urine as a source of biomarkers for prostate cancer that has resulted in a variety of urine tests that are in current clinical use. Importantly, at least three tests have demonstrated high sensitivity (~90%) and negative predictive value (~95%) for clinically significant tumors; however, there has not been widespread adoption of these tests.
Conclusions: Conceptual and methodological advances in the field will help to drive the development of novel urinary tests that in turn may lead to a shift in the clinical paradigm for prostate cancer diagnosis and management.
Conflict of interest statement
Conflict of interest
WJC is a consultant/advisory board member for Beckman Coulter and has received commercial research support from Beckman Coulter, DeCode Genetics, and Ohmx. JL, CPP, JNE and DR declare no conflict of interest.
Figures

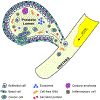
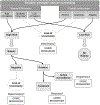

References
-
- Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E: Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. The New England journal of medicine 1987, 317(15):909–916. - PubMed
-
- Lilja H, Ulmert D, Vickers AJ: Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nature Reviews Cancer 2008, 8:268. - PubMed
-
- Blute ML Jr, Abel EJ, Downs TM, Kelcz F, Jarrard DF: Addressing the need for repeat prostate biopsy: new technology and approaches. Nature Reviews Urology 2015, 12:435. - PubMed
-
- Emwas AH, Luchinat C, Turano P, Tenori L, Roy R, Salek RM, Ryan D, Merzaban JS, Kaddurah-Daouk R, Zeri AC et al. : Standardizing the experimental conditions for using urine in NMR-based metabolomic studies with a particular focus on diagnostic studies: a review. Metabolomics : Official journal of the Metabolomic Society 2015, 11(4):872–894. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

