Efficacy of adalimumab as second-line therapy in a pediatric cohort of Crohn's disease patients who failed infliximab therapy: the Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition experience
- PMID: 30655661
- PMCID: PMC6322517
- DOI: 10.2147/BTT.S183088
Efficacy of adalimumab as second-line therapy in a pediatric cohort of Crohn's disease patients who failed infliximab therapy: the Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition experience
Abstract
Background: Adalimumab (Ada) treatment is an available option for pediatric Crohn's disease (CD) and the published experience as rescue therapy is limited.
Objectives: We investigated Ada efficacy in a retrospective, pediatric CD cohort who had failed previous infliximab treatment, with a minimum follow-up of 6 months.
Methods: In this multicenter study, data on demographics, clinical activity, growth, laboratory values (CRP) and adverse events were collected from CD patients during follow-up. Clinical remission (CR) and response were defined with Pediatric CD Activity Index (PCDAI) score ≤10 and a decrease in PCDAI score of ≥12.5 from baseline, respectively.
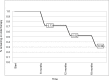
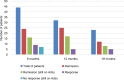
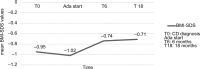
Results: A total of 44 patients were consecutively recruited (mean age 14.8 years): 34 of 44 (77%) had active disease (mean PCDAI score 24.5) at the time of Ada administration, with a mean disease duration of 3.4 (range 0.3-11.2) years. At 6, 12, and 18 months, out of the total of the enrolled population, CR rates were 55%, 78%, and 52%, respectively, with a significant decrease in PCDAI scores (P<0.01) and mean CRP values (mean CRP 5.7 and 2.4 mL/dL, respectively; P<0.01) at the end of follow-up. Steroid-free remission rates, considered as the total number of patients in CR who were not using steroids at the end of this study, were 93%, 95%, and 96% in 44 patients at 6, 12, and 18 months, respectively. No significant differences in growth parameters were detected. In univariate analysis of variables related to Ada efficacy, we found that only a disease duration >2 years was negatively correlated with final PCDAI score (P<0.01). Two serious adverse events were recorded: 1 meningitis and 1 medulloblastoma.
Conclusion: Our data confirm Ada efficacy in pediatric patients as second-line biological therapy after infliximab failure. Longer-term prospective data are warranted to define general effectiveness and safety in pediatric CD patients.
Keywords: adalimumab efficacy; adalimumab safety; infliximab failure; pediatric Crohn’s disease.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



References
-
- Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17(1):423–439. - PubMed
-
- Markowitz J, Hyams J, Mack D, et al. Corticosteroid therapy in the age of infliximab: acute and 1-year outcomes in newly diagnosed children with Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4(9):1124–1129. - PubMed
-
- Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179–1207. - PubMed
-
- Hyams JS, Markowitz J, Wyllie R. Use of infliximab in the treatment of Crohn’s disease in children and adolescents. J Pediatr. 2000;137(2):192–196. - PubMed
-
- Turner D, Grossman AB, Rosh J, et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn’s disease. Am J Gastroenterol. 2007;102(12):2804–2812. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

