Application of built-in adjuvants for epitope-based vaccines
- PMID: 30656066
- PMCID: PMC6336016
- DOI: 10.7717/peerj.6185
Application of built-in adjuvants for epitope-based vaccines
Abstract
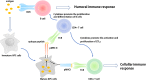
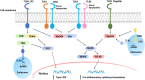
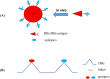
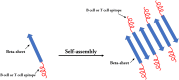
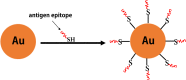
Several studies have shown that epitope vaccines exhibit substantial advantages over conventional vaccines. However, epitope vaccines are associated with limited immunity, which can be overcome by conjugating antigenic epitopes with built-in adjuvants (e.g., some carrier proteins or new biomaterials) with special properties, including immunologic specificity, good biosecurity and biocompatibility, and the ability to vastly improve the immune response of epitope vaccines. When designing epitope vaccines, the following types of built-in adjuvants are typically considered: (1) pattern recognition receptor ligands (i.e., toll-like receptors); (2) virus-like particle carrier platforms; (3) bacterial toxin proteins; and (4) novel potential delivery systems (e.g., self-assembled peptide nanoparticles, lipid core peptides, and polymeric or inorganic nanoparticles). This review primarily discusses the current and prospective applications of these built-in adjuvants (i.e., biological carriers) to provide some references for the future design of epitope-based vaccines.
Keywords: Biological carriers; Built-in adjuvants; Epitope-based vaccines; Nanoparticles.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
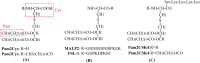

References
-
- Ali M, Pandey RK, Khatoon N, Narula A, Mishra A, Prajapati VK. Exploring dengue genome to construct a multi-epitope based subunit vaccine by utilizing immunoinformatics approach to battle against dengue infection. Scientific Reports. 2017;7(1):9232. doi: 10.1038/s41598-017-09199-w. - DOI - PMC - PubMed
-
- Ambühl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, Nief V, Schellekens C, Sladko K, Roubicek K, Pfister T, Rettenbacher M, Volk H-D, Wagner F, Müller P, Jennings GT, Bachmann MF. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. Journal of Hypertension. 2007;25(1):63–72. doi: 10.1097/HJH.0b013e32800ff5d6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

