Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia
- PMID: 30656180
- PMCID: PMC6331199
- DOI: 10.1002/acn3.660
Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia
Abstract
Objective: Previous studies have demonstrated that suppression of Nrf2 in Friedreich ataxia tissues contributes to excess oxidative stress, mitochondrial dysfunction, and reduced ATP production. Omaveloxolone, an Nrf2 activator and NF-kB suppressor, targets dysfunctional inflammatory, metabolic, and bioenergetic pathways. The dose-ranging portion of this Phase 2 study assessed the safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia patients (NCT02255435).
Methods: Sixty-nine Friedreich ataxia patients were randomized 3:1 to either omaveloxolone or placebo administered once daily for 12 weeks. Patients were randomized in cohorts of eight patients, at dose levels of 2.5-300 mg/day.
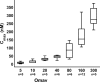
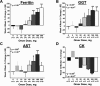
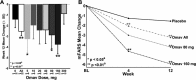
Results: Omaveloxolone was well tolerated, and adverse events were generally mild. Optimal pharmacodynamic changes (noted by changes in ferritin and GGT) were observed at doses of 80 and 160 mg/day. No significant changes were observed in the primary outcome, peak work load in maximal exercise testing (0.9 ± 2.9 W, placebo corrected). At the 160 mg/day dose, omaveloxolone improved the secondary outcome of the mFARS by 3.8 points versus baseline (P = 0.0001) and by 2.3 points versus placebo (P = 0.06). Omaveloxolone produced greater improvements in mFARS in patients that did not have musculoskeletal foot deformity (pes cavus). In patients without this foot deformity, omaveloxolone improved mFARS by 6.0 points from baseline (P < 0.0001) and by 4.4 points versus placebo (P = 0.01) at the 160 mg/day.
Interpretation: Treatment of Friedreich ataxia patients with omaveloxolone at the optimal dose level of 160 mg/day appears to improve neurological function. Therefore, omaveloxolone treatment is being examined in greater detail at 150 mg/day for Friedreich ataxia.
Figures


References
-
- Lynch DR, Farmer JM, Balcer LJ, Wilson RB. Friedreich ataxia: effects of genetic understanding on clinical evaluation and therapy. Arch Neurol 2002;59:743–747. - PubMed
-
- Pandolfo M. Friedreich ataxia. Arch Neurol 2008;65:1296–1303. - PubMed
-
- Schulz JB, Boesch S, Bürk K, et al. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol 2009;5:222–234. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

