IVIg-induced plasmablasts in patients with Guillain-Barré syndrome
- PMID: 30656191
- PMCID: PMC6331722
- DOI: 10.1002/acn3.687
IVIg-induced plasmablasts in patients with Guillain-Barré syndrome
Abstract
Objective: The Guillain-Barré syndrome (GBS) is an acute, immune-mediated disease of peripheral nerves. Plasmablasts and plasma cells play a central role in GBS by producing neurotoxic antibodies. The standard treatment for GBS is high-dose intravenous immunoglobulins (IVIg), however the working mechanism is unknown and the response to treatment is highly variable. We aimed to determine whether IVIg changes the frequency of B-cell subsets in patients with GBS.
Methods: Peripheral blood mononuclear cells were isolated from 67 patients with GBS before and/or 1, 2, 4, and 12 weeks after treatment with high-dose IVIg. B-cell subset frequencies were determined by flow cytometry and related to serum immunoglobulin levels. Immunoglobulin transcripts before and after IVIg treatment were examined by next-generation sequencing. Antiglycolipid antibodies were determined by ELISA.
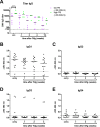
Results: Patients treated with IVIg demonstrated a strong increase in plasmablasts, which peaked 1 week after treatment. Flow cytometry identified a relative increase in IgG2 plasmablasts posttreatment. Within IGG sequences, dominant clones were identified which were also IGG2 and had different immunoglobulin sequences compared to pretreatment samples. High plasmablast frequencies after treatment correlated with an increase in serum IgG and IgM, suggesting endogenous production. Patients with a high number of plasmablasts started to improve earlier (P = 0.015) and were treated with a higher dose of IVIg.
Interpretation: High-dose IVIg treatment alters the distribution of B-cell subsets in the peripheral blood of GBS patients, suggesting de novo (oligo-)clonal B-cell activation. Very high numbers of plasmablasts after IVIg therapy may be a potential biomarker for fast clinical recovery.
Figures





References
-
- Willison HJ, Jacobs BC, van Doorn PA. Guillain‐Barré syndrome. Lancet 2016;388:717–727. - PubMed
-
- McGonigal R, Rowan EG, Greenshields KN, et al. Anti‐GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain 2010;133:1944–1960. - PubMed
-
- van der Meché FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain‐Barré syndrome. Dutch Guillain‐Barré Study Group. N Engl J Med 1992;326:1123–1129. - PubMed
-
- van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain‐Barré syndrome. Lancet Neurol 2008;7:939–950. - PubMed
-
- Kuitwaard K, de Gelder J, Tio‐Gillen AP, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain‐Barré syndrome. Ann Neurol 2009;66:597–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
