Nuclear ErbB-2: a Novel Therapeutic Target in ErbB-2-Positive Breast Cancer?
- PMID: 30656558
- PMCID: PMC10355700
- DOI: 10.1007/s12672-018-0356-3
Nuclear ErbB-2: a Novel Therapeutic Target in ErbB-2-Positive Breast Cancer?
Abstract
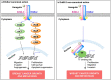
Membrane overexpression of ErbB-2 (MErbB-2), a member of the ErbB family of receptor tyrosine kinases, occurs in 15-20% of breast cancers (BC) and constitutes a therapeutic target in this BC subtype (ErbB-2-positive). Although MErbB-2-targeted therapies have significantly improved patients' clinical outcome, resistance to available drugs is still a major issue in the clinic. Lack of accurate biomarkers for predicting responses to anti-ErbB-2 drugs at the time of diagnosis is also an important unresolved issue. Hence, a better understanding of the ErbB-2 signaling pathway constitutes a critical task in the battle against BC. In its canonical mechanism of action, MErbB-2 activates downstream signaling pathways, which transduce its proliferative effects in BC. The dogma of ErbB-2 mechanism of action has been challenged by the demonstration that MErbB-2 migrates to the nucleus, where it acts as a transcriptional regulator. Accumulating findings demonstrate that nuclear ErbB-2 (NErbB-2) is involved in BC growth and metastasis. Emerging evidence also reveal a role of NErbB-2 in the response to available anti-MErbB-2 agents. Here, we will review NErbB-2 function in BC and will particularly discuss the role of NErbB-2 as a novel target for therapy in ErbB-2-positive BC.
Keywords: Breast cancer; ErbB-2 signaling pathway; Metastasis; Nuclear ErbB-2; Response to ErbB-2-targeted therapies; Transcriptional coactivator.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. - DOI - PubMed
-
- Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, Cristofanilli M, Arun B, Esmaeli B, Fritsche HA, Sneige N, Smith TL, Hortobagyi GN. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(7):1800–1808. doi: 10.1200/JCO.2002.07.058. - DOI - PubMed
-
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- IDB/PICT 2015-1587/National Agency of Scientific Promotion of Argentina (ANPCyT)/International
- IDB/ PICT 2012-668/National Agency of Scientific Promotion of Argentina (ANPCyT)/International
- PID 2012-066/National Agency of Scientific Promotion of Argentina (ANPCyT)/International
- Not available/Fondation Nelia et Amadeo Barletta/International
- Not available/National Institute of Cancer from Argentina/International
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

