Monitoring the Efficacy and Safety of Artemisinin-Based Combination Therapies: A Review and Network Meta-analysis of Antimalarial Therapeutic Efficacy Trials in Cameroon
- PMID: 30656608
- PMCID: PMC6380963
- DOI: 10.1007/s40268-018-0259-3
Monitoring the Efficacy and Safety of Artemisinin-Based Combination Therapies: A Review and Network Meta-analysis of Antimalarial Therapeutic Efficacy Trials in Cameroon
Abstract
Introduction: Artemisinin-based combination therapies (ACTs) are the first-line antimalarial drugs used to treat uncomplicated Plasmodium falciparum alaria in many endemic countries worldwide. The present work reviewed the therapeutic efficacy of ACT in Cameroon more than 10 years after the initial change in national drug policy in 2004.
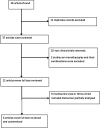
Methods: A PubMed literature search was performed to analyse clinical trials conducted in Cameroon from 2001 to May 2017. Clinical studies that evaluated ACT for the treatment of uncomplicated falciparum malaria in children or adults, and reported efficacy and/or safety, were included. In addition, a small network meta-analysis (NMA) with a frequentist approach was performed.
Results: Six papers were selected from 48 articles screened and were full-text reviewed. The efficacy of both artemether-lumefantrine (AL) and artesunate-amodiaquine (ASAQ) ranged from moderate to high, with polymerase chain reaction-corrected cure rates ranging from 96.7 to 100% and 88.2 to 100%, respectively, in per-protocol analysis, and 86.2 to 96.7% and 74.0 to 90.6%, respectively, in intention-to-treat analysis. The malaria evidence network suggested that AL and ASAQ efficacies were comparable. The highest day 3 parasite positivity rate was 8.2% for ASAQ and 4% for AL. A novel ACT, artesunate-atovaquoneproguanil (ASATPG) was tested once and showed a cure rate of 100%. Based on an ITT approach, the NMA revealed that AL was more efficacious than ASAQ, but the difference was not statistical significant (706 participants, three randomised clinical trials (RCT); OR 1.25, 95%CI 0.78-2.00). Adverse events ranged from mild to moderate severity but were not directly attributed to drug intake.
Conclusion: ACTs are still effective and safe in Cameroon; however, there are insufficient data on their efficacy, safety and tolerability, therefore more RCTs should be conducted, including novel ACTs.
Conflict of interest statement
Conflict of Interest
Solange Whegang Youdom, Andreas Chiabi and Leonardo K. Basco declare that they have no conflicts of interest.
Availability of Data and Materials
Data used in this study can be found in the text file and in electronic supplementary files 1, 2, 3 and 4.
Figures


Similar articles
-
Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2022 Jun 21;6(6):CD006404. doi: 10.1002/14651858.CD006404.pub4. Cochrane Database Syst Rev. 2022. PMID: 35726133 Free PMC article.
-
Efficacy of artemether-lumefantrine, artesunate-amodiaquine, dihydroartemisinin-piperaquine and artesunate-pyronaridine for the treatment of uncomplicated Plasmodium falciparum malaria in Mozambique, 2022.Malar J. 2025 Jul 14;24(1):231. doi: 10.1186/s12936-025-05473-9. Malar J. 2025. PMID: 40660279 Free PMC article. Clinical Trial.
-
Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2014 Jan 20;2014(1):CD010927. doi: 10.1002/14651858.CD010927. Cochrane Database Syst Rev. 2014. PMID: 24443033 Free PMC article.
-
Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2014 Mar 4;(3):CD006404. doi: 10.1002/14651858.CD006404.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Jan 08;1:CD006404. doi: 10.1002/14651858.CD006404.pub3. PMID: 24596021 Free PMC article. Updated.
-
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission.Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. PMID: 24979199 Free PMC article. Updated.
Cited by
-
Molecular Surveillance of Artemisinin-Based Combination Therapies Resistance in Plasmodium falciparum Parasites from Bioko Island, Equatorial Guinea.Microbiol Spectr. 2022 Jun 29;10(3):e0041322. doi: 10.1128/spectrum.00413-22. Epub 2022 Jun 7. Microbiol Spectr. 2022. PMID: 35670601 Free PMC article.
-
Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Mali: a systematic review and meta-analysis.Malar J. 2021 Aug 30;20(1):356. doi: 10.1186/s12936-021-03890-0. Malar J. 2021. PMID: 34461901 Free PMC article.
-
Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination.Parasit Vectors. 2019 Oct 26;12(1):501. doi: 10.1186/s13071-019-3753-8. Parasit Vectors. 2019. PMID: 31655608 Free PMC article. Review.
-
Novel Plasmodium falciparum Kelch13 polymorphisms in Cameroon with structural and physicochemical impact.Antimicrob Agents Chemother. 2025 May 7;69(5):e0088424. doi: 10.1128/aac.00884-24. Epub 2025 Apr 14. Antimicrob Agents Chemother. 2025. PMID: 40227034 Free PMC article.
-
Quantum Chemical Lipophilicities of Antimalarial Drugs in Relation to Terminal Half-Life.ACS Omega. 2020 Mar 23;5(12):6500-6515. doi: 10.1021/acsomega.9b04140. eCollection 2020 Mar 31. ACS Omega. 2020. PMID: 32258886 Free PMC article.
References
-
- World Health Organization . World malaria report 2017. Geneva: World Health Organization; 2017.
-
- World Health Organization . Antimalarial drug combination therapy. Report of a WHO technical consultation. Geneva: World Health Organization; 2001.
-
- World Health Organization . Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization; 2003.
-
- World Health Organization . Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization; 2009.
-
- World Health Organization . Guidelines for the treatment of malaria. 3. Geneva: World Health Organization; 2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

