The impact of in utero HIV exposure on gut microbiota, inflammation, and microbial translocation
- PMID: 30657007
- PMCID: PMC6748604
- DOI: 10.1080/19490976.2018.1560768
The impact of in utero HIV exposure on gut microbiota, inflammation, and microbial translocation
Abstract
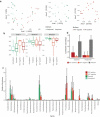
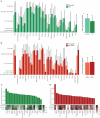
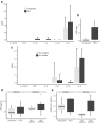
HIV-exposed but uninfected (HEU) children represent a growing population and show a significantly higher number of infectious diseases, several immune alterations, compromised growth, and increased mortality rates when compared to HIV-unexposed children. Considering the impact that the gut microbiota has on general host homeostasis and immune system development and modulation, we hypothesized that HEU children present altered gut microbiota that is linked to the increased morbidity and the immune system disorders faced by them. Our experiments revealed no differences in beta and alpha diversity of the gut microbiota between HEU and unexposed children or between HIV-infected and uninfected mothers. However, there were differences in the abundance of several taxa from the gut microbiota between HEU and unexposed children and between HIV-infected and uninfected mothers. Functional prediction based on 16S rRNA sequences also indicated differences between HEU and unexposed children and between infected and uninfected mothers. In addition, we detected no differences between HEU and unexposed children in relation to weight, weight-for-age z scores, albumin serum levels, or microbial translocation and inflammation markers. In summary, HIV-infected mothers and their HIV-exposed children present alterations in the abundance of several taxa in the gut microbiome and the predicted functional metagenome when compared to uninfected mothers and unexposed children. Knowledge about the gut microbiome of HEU children in different settings is essential in order to determine better treatments for this susceptible population.
Keywords: 16S rRNA; HIV; children; exposed uninfected; gut microbiota; inflammation; microbial translocation.
Figures

Similar articles
-
Evolution of the Gut Microbiome in HIV-Exposed Uninfected and Unexposed Infants during the First Year of Life.mBio. 2022 Oct 26;13(5):e0122922. doi: 10.1128/mbio.01229-22. Epub 2022 Sep 8. mBio. 2022. PMID: 36073815 Free PMC article.
-
Dynamics of the infant gut microbiota in the first 18 months of life: the impact of maternal HIV infection and breastfeeding.Microbiome. 2022 Apr 12;10(1):61. doi: 10.1186/s40168-022-01230-1. Microbiome. 2022. PMID: 35414043 Free PMC article.
-
Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months.Pediatrics. 2017 Oct;140(4):e20170988. doi: 10.1542/peds.2017-0988. Epub 2017 Sep 14. Pediatrics. 2017. PMID: 28912368 Free PMC article.
-
Health and survival of HIV perinatally exposed but uninfected children born to HIV-infected mothers.Curr Opin HIV AIDS. 2016 Sep;11(5):465-476. doi: 10.1097/COH.0000000000000300. Curr Opin HIV AIDS. 2016. PMID: 27716731 Review.
-
Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis.Pediatrics. 2018 Feb;141(2):e20172888. doi: 10.1542/peds.2017-2888. Pediatrics. 2018. PMID: 29374109 Free PMC article.
Cited by
-
Factors influencing maternal microchimerism throughout infancy and its impact on infant T cell immunity.J Clin Invest. 2022 Jul 1;132(13):e148826. doi: 10.1172/JCI148826. J Clin Invest. 2022. PMID: 35550376 Free PMC article.
-
Evolution of the Gut Microbiome in HIV-Exposed Uninfected and Unexposed Infants during the First Year of Life.mBio. 2022 Oct 26;13(5):e0122922. doi: 10.1128/mbio.01229-22. Epub 2022 Sep 8. mBio. 2022. PMID: 36073815 Free PMC article.
-
The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis.Autoimmune Dis. 2020 Jun 25;2020:5793817. doi: 10.1155/2020/5793817. eCollection 2020. Autoimmune Dis. 2020. PMID: 32789026 Free PMC article. Review.
-
Early changes in the gut microbiome among HIV-infected Individuals in Uganda initiating daily TMP/SMX.medRxiv [Preprint]. 2024 Oct 7:2024.10.07.24315002. doi: 10.1101/2024.10.07.24315002. medRxiv. 2024. PMID: 39417122 Free PMC article. Preprint.
-
Review on the Alteration of Gut Microbiota: The Role of HIV Infection and Old Age.AIDS Res Hum Retroviruses. 2020 Jul;36(7):556-565. doi: 10.1089/AID.2019.0282. Epub 2020 May 18. AIDS Res Hum Retroviruses. 2020. PMID: 32323556 Free PMC article. Review.
References
-
- UNAIDS On the fast-track to an AIDS-free generation. Geneva: 2016. [accessed 2017 December18] http://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
